Treating MDD: How Brintellix® (vortioxetine) improves a broad range of major depressive disorder (MDD) symptoms [1,2]
Two meta-analysis demonstrate the broad beneficial effects of Brintellix® on emotional, physical and cognitive symptoms1,2
As a complex condition involving a variety of symptoms, MDD requires a comprehensive treatment approach for patients to achieve functional recovery.3-5
Brintellix® is a multi-modal antidepressant that has demonstrable efficacy across a broad range of MDD symptoms, as measured by two common depression rating scales – the MADRS1 and HAM-D2.
Explore the data from two studies assessing the broad efficacy of Brintellix® below.
Brintellix® demonstrates efficacy across a broad range of MDD symptoms, with a clear dose-response 1
In this meta-analysis by Thase ME et al. (2016), 11 placebo-controlled, short-term(6-8 weeks) trials were studied to assess the efficacy and safety of Brintellix®. It showed that Brintellix® significantly improves all symptoms of depression assessed by the MADRS scale, compared with placebo, in a dose dependent manner.'
Significant differences vs. placebo in all MADRS single items in a short-term meta-analysis of 11 controlled studies (6-8 weeks) (FAS, MMRM)1
In this meta-analysis by Thase ME et al. (2016), 11 placebo-controlled, short-term(6-8 weeks) trials were studied to assess the efficacy and safety of Brintellix®. It showed that Brintellix® significantly improves all symptoms of depression assessed by the MADRS scale, compared with placebo, in a dose dependent manner.'
Significant differences vs. placebo in all MADRS single items in a short-term meta-analysis of 11 controlled studies (6-8 weeks) (FAS, MMRM)1
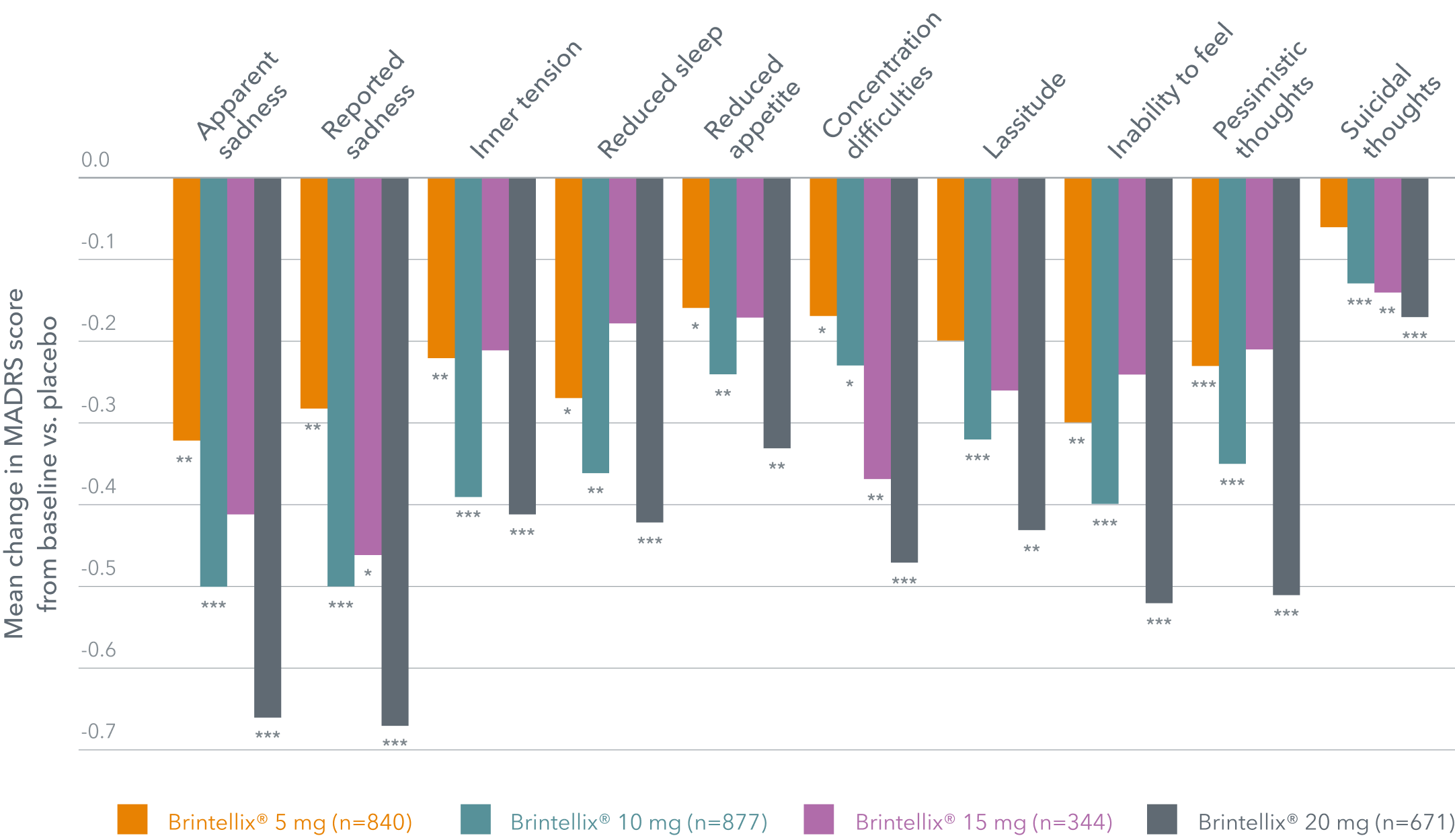
Adapted from: Thase ME et al. 2016.
t Inability to feel representing subjective experience of reduced interest in the surroundings or activities that normally give pleasure; the ability to react with adequate emotion to circumstances or people is reduced,’ i.e., emotional blunting.
*p<0.05; **p<0.01; ***p<0.001
The starting and recommended dose of Brintellix® is 10 mg/day. Depending on individual patient response, the dose can be increased to a maximum of Brintellix® 20 mg/day or decreased to a minimum of Brintellix® 5 mg/day, if needed.6
Brintellix® significantly improves physical symptoms of MDD2
HAM-D single items in a meta-analysis of 5 controlled studies (6-8 weeks)2
Adapted from: Thase ME et al. 2016.
t Inability to feel representing subjective experience of reduced interest in the surroundings or activities that normally give pleasure; the ability to react with adequate emotion to circumstances or people is reduced,’ i.e., emotional blunting.
*p<0.05; **p<0.01; ***p<0.001
The starting and recommended dose of Brintellix® is 10 mg/day. Depending on individual patient response, the dose can be increased to a maximum of Brintellix® 20 mg/day or decreased to a minimum of Brintellix® 5 mg/day, if needed.6
Brintellix® significantly improves physical symptoms of MDD2
HAM-D single items in a meta-analysis of 5 controlled studies (6-8 weeks)2
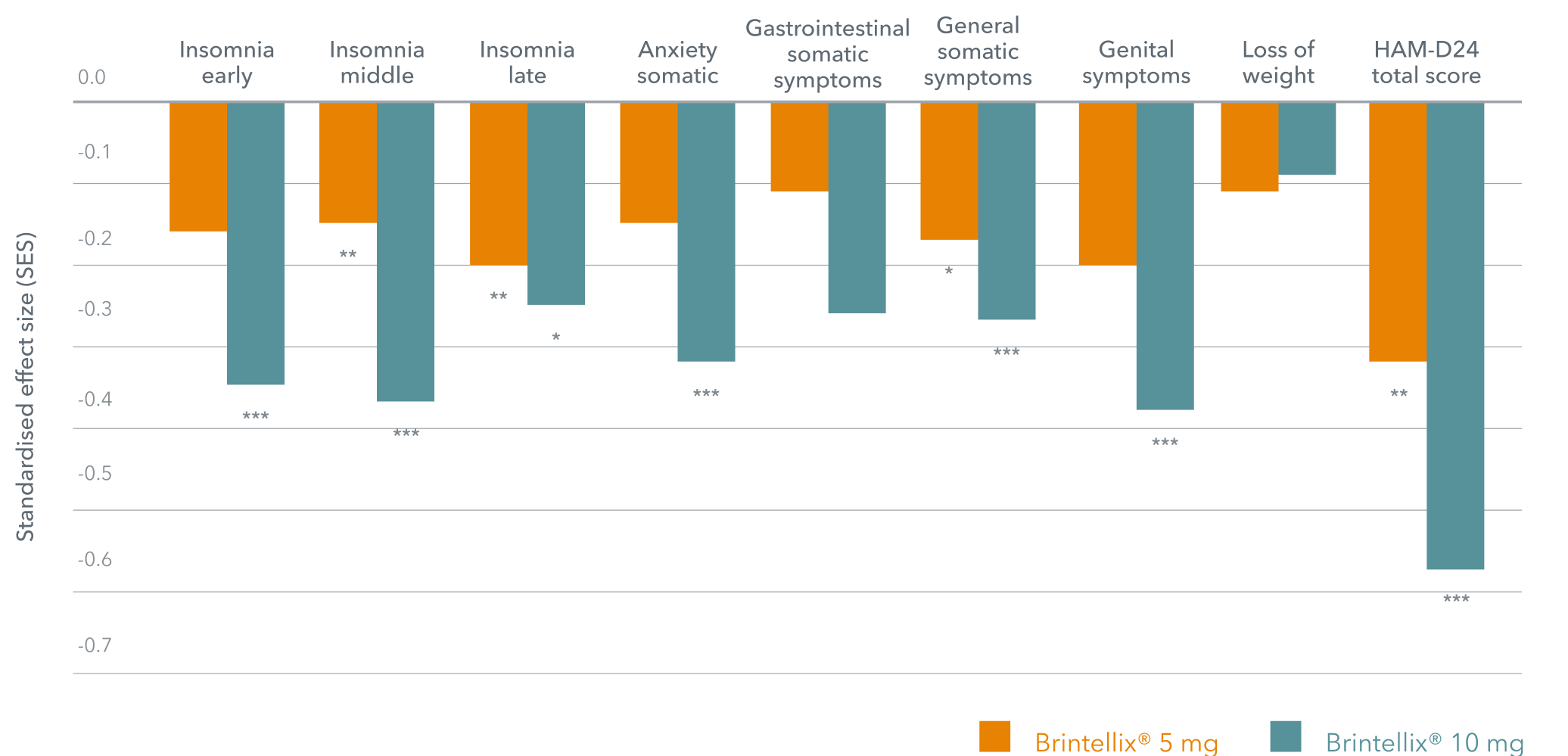
Adapted from Christensen MC et al. 2018.
*p<0.05; **p<0.01; ***p<0.001.
Meta-analysis included five short-term multinational double-blind placebo-controlled studies including a total of 2,105 adult outpatients with a major depressive episode ≥3 months duration. Only patients treated with a dose of either Brintellix® 5 mg or 10 mg, or placebo, were included in this analysis.
In this meta-analysis, Christensen MC et al. (2018) assessed the efficacy of Brintellix® on the physical symptoms of depression via the HAM-D, a depression rating scale that allows the assessment of a broad range of physical symptoms.2
The analysis examined five short-term placebo-controlled studies in patients with MDD treated with either Brintellix® 5 mg or 10 mg or placebo, demonstrating that Brintellix® significantly improved most physical symptoms of depression, as measured by the HAM-D.2
Adapted from Christensen MC et al. 2018.
*p<0.05; **p<0.01; ***p<0.001.
Meta-analysis included five short-term multinational double-blind placebo-controlled studies including a total of 2,105 adult outpatients with a major depressive episode ≥3 months duration. Only patients treated with a dose of either Brintellix® 5 mg or 10 mg, or placebo, were included in this analysis.
In this meta-analysis, Christensen MC et al. (2018) assessed the efficacy of Brintellix® on the physical symptoms of depression via the HAM-D, a depression rating scale that allows the assessment of a broad range of physical symptoms.2
The analysis examined five short-term placebo-controlled studies in patients with MDD treated with either Brintellix® 5 mg or 10 mg or placebo, demonstrating that Brintellix® significantly improved most physical symptoms of depression, as measured by the HAM-D.2
Abbreviations:
HAM-D, Hamilton depression rating scale; MADRS, Montgomery-Åsberg depression rating scale; MDD,
major depressive disorder; MDE, major depressive episode.
Abbreviations:
HAM-D, Hamilton depression rating scale; MADRS, Montgomery-Åsberg depression rating scale; MDD,
major depressive disorder; MDE, major depressive episode.