The long-term efficacy of Brintellix® (vortioxetine) in patients with major depressive disorder (MDD) [1]
As many as 1 in 4 people who recover from a major depressive episode (MDE) will have a recurrence within the first year.2 Additionally, a greater number of previous episodes, a longer duration of a MDE and more severe depressive symptoms are typically predictive of future recurrences.3
To prevent relapse and recurrence, long-term treatment of at least six months following remission of the acute depressive episode is recommended.
Data from five long-term, open-label, flexible-dose extension studies assessed whether improvements in depressive symptoms in MDD continue after acute (six-to-eight weeks) Brintellix® treatment.1
See the findings below.
Brintellix® (5-20 mg) delivers sustained relief from symptoms of MDD† over the long-term.1,5
Change in MADRS total score for patients previously treated with Brintellix® 5-20 mg/day in controlled trials (acute) who continued treatment in open-label extension studies over 52 weeks1
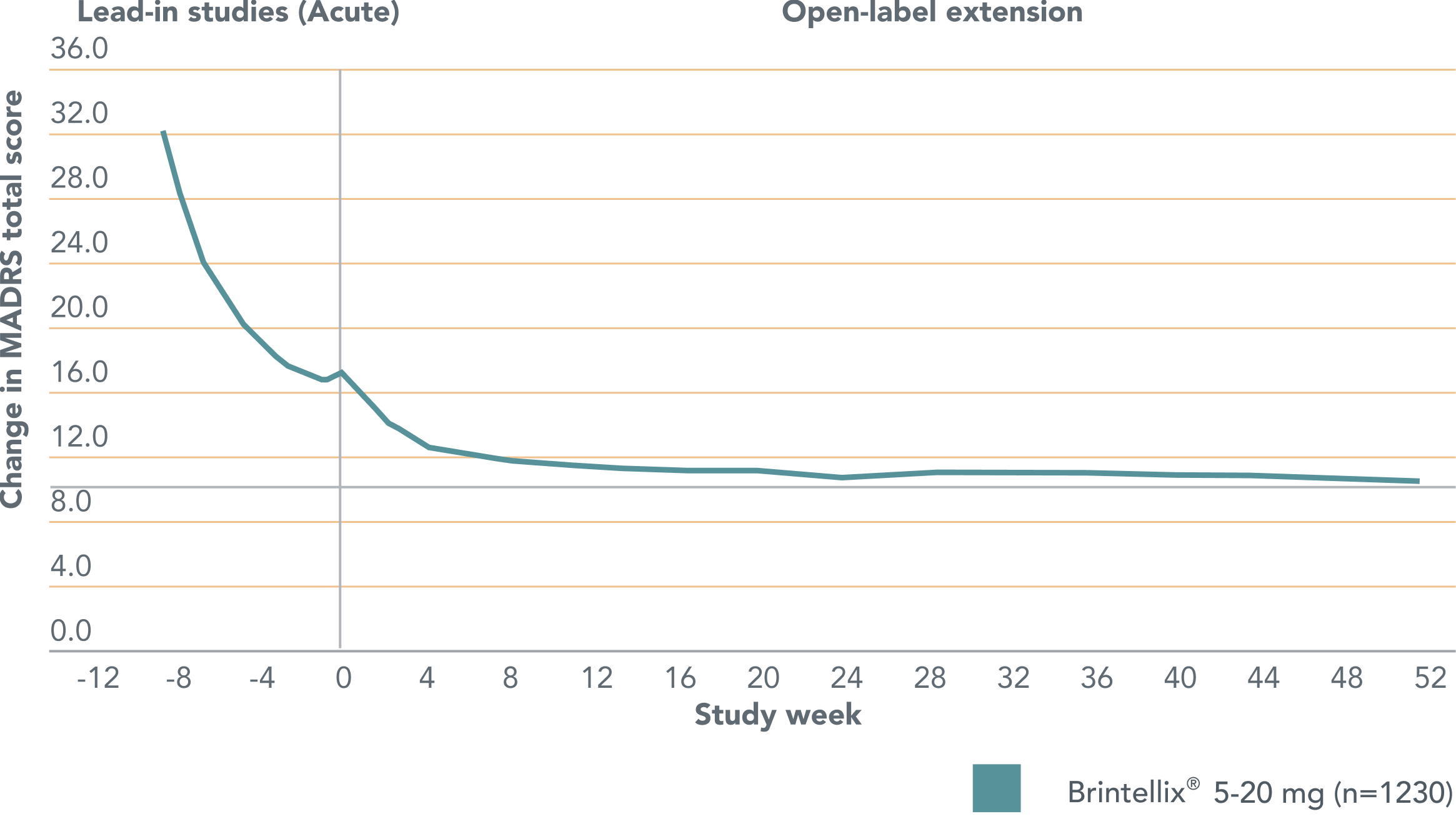
Adapted from Vieta E et al. 2017.
The horizontal dashed line indicates the cut point for remission (defined by MADRS total score ≤10).
The vertical dashed line represents start of treatment in the extension study.
Improvements in MDD symptoms† seen with Brintellix® during the short-term (6-8 weeks) studies continued at the start of the extension study and were maintained with continuous treatment over 52 weeks.1
Adapted from Vieta E et al. 2017.
The horizontal dashed line indicates the cut point for remission (defined by MADRS total score ≤10).
The vertical dashed line represents start of treatment in the extension study.
Improvements in MDD symptoms† seen with Brintellix® during the short-term (6-8 weeks) studies continued at the start of the extension study and were maintained with continuous treatment over 52 weeks.1

Brintellix® was shown to be generally well tolerated over the long term1 and had a safety profile consistent with previous clinical studies.5,6 The most commonly reported treatment-emergent adverse event (TEAE) was nausea (16.6%). Other TEAEs with an incidence of ≥5% included headache (12.9%), nasopharyngitis (9.4%), diarrhea (6.4%) and weight increase (5.3%).1 The rate of withdrawal during the long-term extension due to any TEAE was low (7.8%).1
Brintellix® was shown to be generally well tolerated over the long term1 and had a safety profile consistent with previous clinical studies.5,6 The most commonly reported treatment-emergent adverse event (TEAE) was nausea (16.6%). Other TEAEs with an incidence of ≥5% included headache (12.9%), nasopharyngitis (9.4%), diarrhea (6.4%) and weight increase (5.3%).1 The rate of withdrawal during the long-term extension due to any TEAE was low (7.8%).1

† As measured by MADRS.
§ Based on incidence of discontinuation symptoms (as measured by the DESS scale) and withdrawal rates due to TEAEs.5,6
Abbreviations:
MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; MDE, major depressive episode; TEAE, treatment-emergent adverse event.
† As measured by MADRS.
§ Based on incidence of discontinuation symptoms (as measured by the DESS scale) and withdrawal rates due to TEAEs.5,6
Abbreviations:
MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; MDE, major depressive episode; TEAE, treatment-emergent adverse event.

