Increasing doses of SSRIs were associated with a linear increase in adverse events, but response rates were not
A comprehensive dose-response meta-analysis of the most commonly prescribed antidepressants for major depression was performed1. For SSRIsa, the probability of responseto treatment increased up to doses between 20 mg and 40 mg of fluoxetine equivalents, with no further increase or even a slight decrease at higher doses within the licensed dose range up to 80 mg. Dropouts due to adverse events
(AEs) showed an exponential increase with an increase in SSRI dose1.
Meta-analysis: Dose-outcome relationships for SSRIs (99 treatment groups)
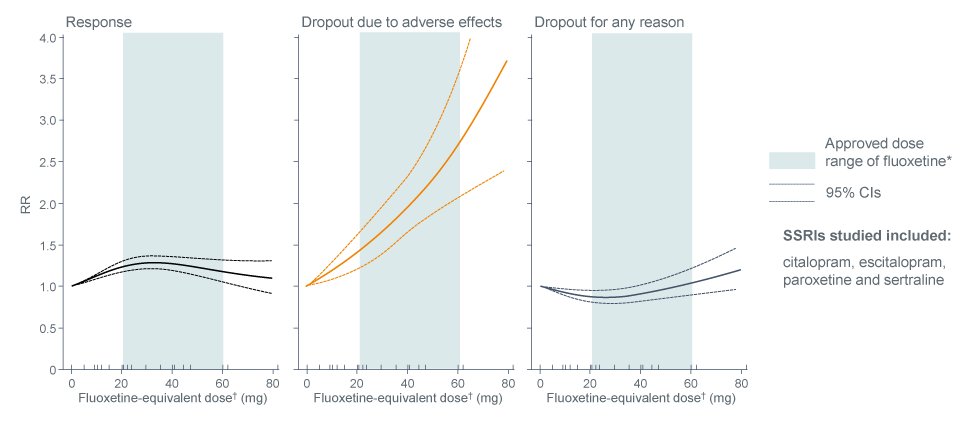
# Obtained from the Summary of Product Characteristics for fluoxetine;
† Doses of citalopram, escitalopram, paroxetine and sertraline were converted to fluoxetine equivalents.
# Obtained from the Summary of Product Characteristics for fluoxetine;
† Doses of citalopram, escitalopram, paroxetine and sertraline were converted to fluoxetine equivalents.
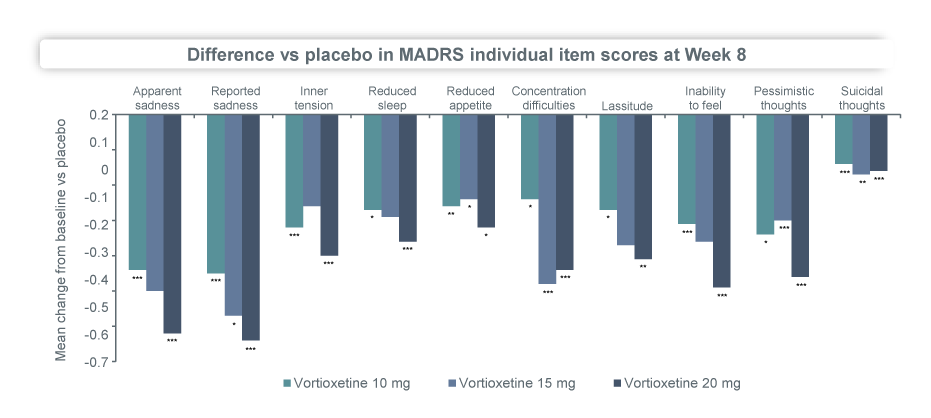
In a pooled analysis of 6 short-term randomised, placebo-controlled studies of 3032 MDD patients, Brintellix® (vortioxetine) demonstrated a clear dose-response relationship in terms of improvement in MADRS scores, with patients on the higher dose of Brintellix® (20 mg/day) experiencing greater reductions in depressive symptoms compared to the lower dose of 10 mg/day2. By Week 8, up to 64.3% of the patients had titrated up to the high dose of Brintellix®2. The incidence of AEs was comparable between the groups of patients on 20 mg/day vs 10 mg/day2.
Brintellix® improves all symptoms of MDD in adults as assessed by MADRS in a dose-dependant manner2
Incidence of adverse events was not increased in patients who received Brintellix® at the high dose of 20 mg/day vs the lower dose of 10 mg/day?
Incidence of adverse events was not increased in patients who received Brintellix® at the high dose of 20 mg/day vs the lower dose of 10 mg/day?
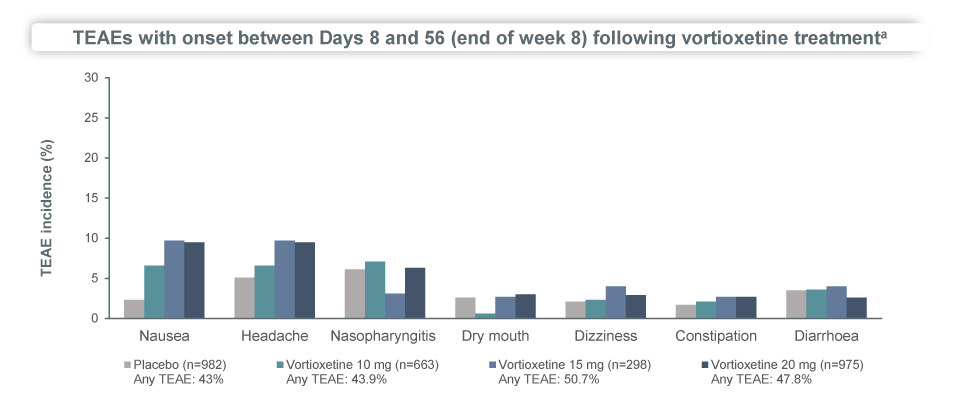
In summary, the pool analysis findings confirm that the high dose of Brintellix® 20 mg/day provides greater efficacy than the lower dose of 10 mg/day in treating symptoms of MDD without compromising the safety and tolerability profile of the antidepressant treatment.2
In summary, the pool analysis findings confirm that the high dose of Brintellix® 20 mg/day provides greater efficacy than the lower dose of 10 mg/day in treating symptoms of MDD without compromising the safety and tolerability profile of the antidepressant treatment.2
a SSRIs study included citalopram, escitalopram, paroxetine and sertraline;
b Treatment response is defined as 50% or greater reduction in depression severity;
c Vortioxetine doses included in the study were 5, 10, 15 and 20mg;
d TEAEs by Medical Dictionary for Regulatory Activities preferred terms with incidence ≥3% in ≥1 group;
* p<0.05 vs placebo;
** p<0.01 vs placebo;
*** p<0.001 vs placebo;
Abbreviations:
AE, adverse event; Cl, confidence interval; MADRS, Montgomery-Asberg Depression Rating Scale; MDD, major depressive disorder; RR, risk ratio; SSRI, selective serotonin reuptake inhibitor
a SSRIs study included citalopram, escitalopram, paroxetine and sertraline;
b Treatment response is defined as 50% or greater reduction in depression severity;
c Vortioxetine doses included in the study were 5, 10, 15 and 20mg;
d TEAEs by Medical Dictionary for Regulatory Activities preferred terms with incidence ≥3% in ≥1 group;
* p<0.05 vs placebo;
** p<0.01 vs placebo;
*** p<0.001 vs placebo;
Abbreviations:
AE, adverse event; Cl, confidence interval; MADRS, Montgomery-Asberg Depression Rating Scale; MDD, major depressive disorder; RR, risk ratio; SSRI, selective serotonin reuptake inhibitor

