Head-to-head study: Brintellix® (vortioxetine) vs the SNRI venlafaxine[1]
A highly important issue in the treatment of MDD in clinical practice is to know which antidepressants to use and how to proceed in cases of non-response.1 Head-to-head studies can give a better idea of the treatments’ relative efficacies.1
Brintellix® has comparable efficacy‡ but greater tolerability than venlafaxine in a head-to-head study in patients with acute MDD1
Mean change in MADRS total score from baseline to week 8 (FAS, OC)
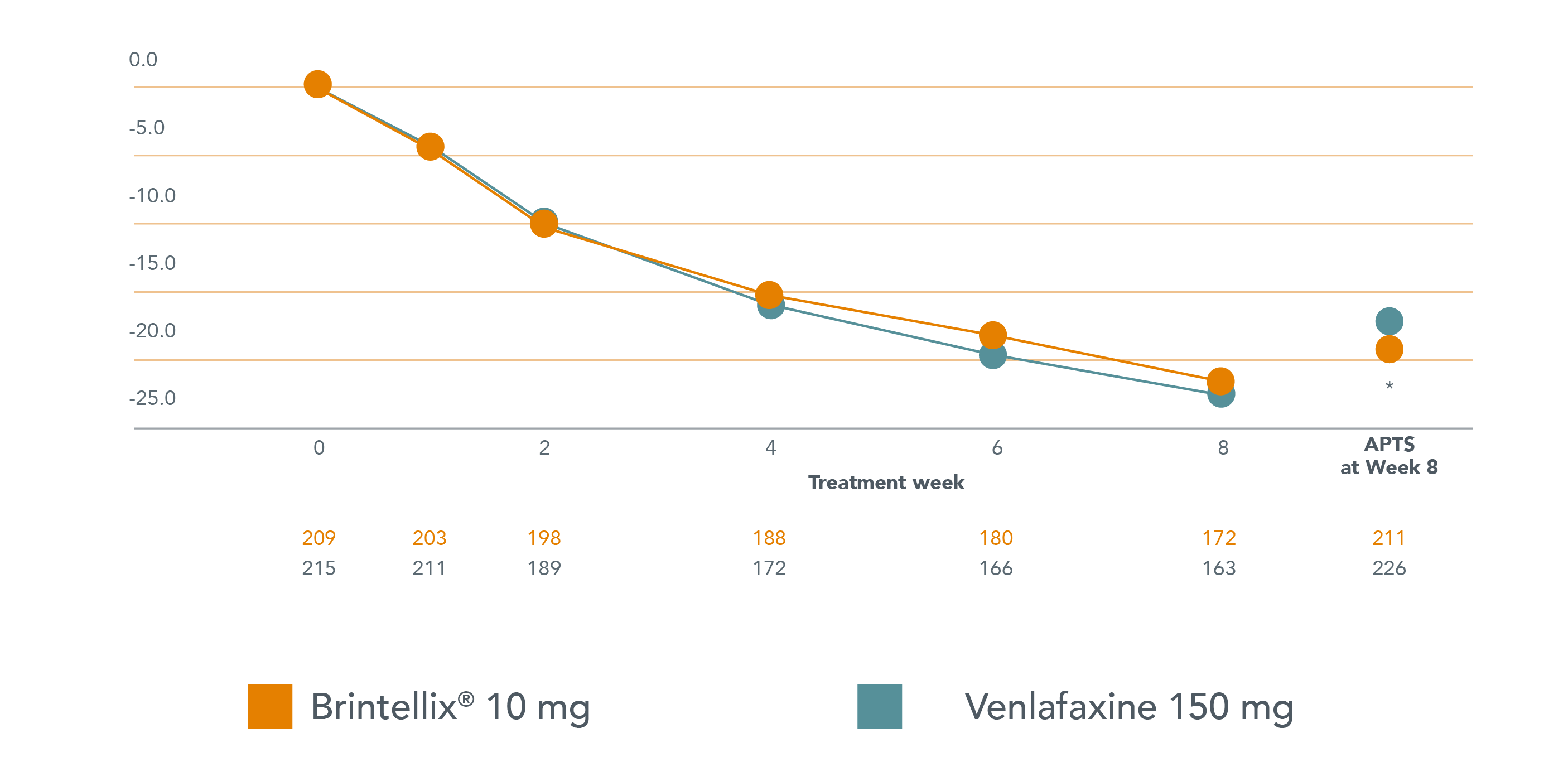
Adapted from Wang G et al. 2015.
Adapted from Wang G et al. 2015.

6.6% of patients treated with Brintellix® and 13.7% of patients treated with venlafaxine, respectively, withdrew from the study due to TEAEs.1 Adverse events with an incidence of ≥5% for both treatments included nausea, dizziness, headache and dry mouth.
Additionally, patients treated with venlafaxine further reported accidental overdose, decreased appetite, constipation and insomnia (≥5% incidence).1
6.6% of patients treated with Brintellix® and 13.7% of patients treated with venlafaxine, respectively, withdrew from the study due to TEAEs.1 Adverse events with an incidence of ≥5% for both treatments included nausea, dizziness, headache and dry mouth.
Additionally, patients treated with venlafaxine further reported accidental overdose, decreased appetite, constipation and insomnia (≥5% incidence).1
‡ As measured with MADRS total score.
* p<0.05 vs. venlafaxine . APTS, all patients treated set - this includes all patients in the study that received at least one dose of treatment.
§Statistically and clinically significant advantage for Brintellix® compared to venlafaxine XR of 1.9 points on the MADRS (p<0.05).1 When comparing two active treatments, a 1-point difference in MADRS total score is considered clinically relevant.2
Abbreviations:
APTS, all patients treated set; FAS, full analysis set; MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; OC, observed caases; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TEAE, treatment-emergent adverse event; XR, extended release.
‡ As measured with MADRS total score.
* p<0.05 vs. venlafaxine . APTS, all patients treated set - this includes all patients in the study that received at least one dose of treatment.
§Statistically and clinically significant advantage for Brintellix® compared to venlafaxine XR of 1.9 points on the MADRS (p<0.05).1 When comparing two active treatments, a 1-point difference in MADRS total score is considered clinically relevant.2
Abbreviations:
APTS, all patients treated set; FAS, full analysis set; MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; OC, observed caases; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TEAE, treatment-emergent adverse event; XR, extended release.

