Clinical benefits of early use of Brintellix ® 20 mg 1
An early response during the first 2 weeks of treatment can predict a positive outcome for sustained response in patients with major depressive disorder (MDD).†2 A recent meta-analysis explored the clinical relevance of early and sustained improvement in depressive symptoms with Brintellix® (vortioxetine) 20 mg compared to 10 mg.
Brintellix® 20 mg allowed significantly more patients with MDD to achieve symptomatic response‡ compared to those receiving 10 mg.1 Patients treated with Brintellix® 20 mg had a significantly shorter time to symptomatic response compared to Brintellix® 10 mg or placebo.1
Proportion of patients achieving MADRS response‡1
(≥50% decrease in MADRS total score from baseline)
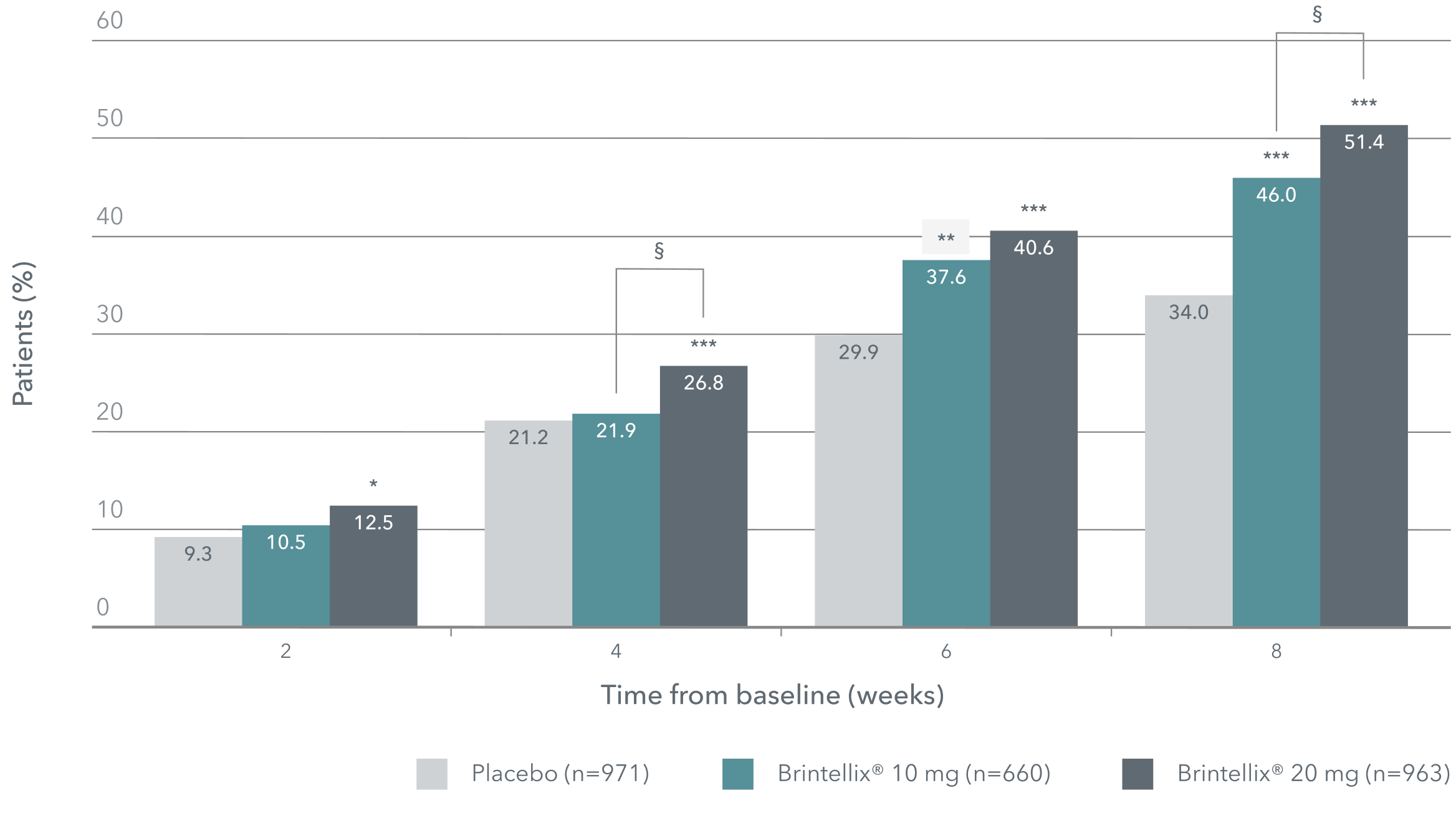
Adapted from Christensen MC et al. 2023.
‡Meta-analysis of six 8-week, fixed-dose, placebo-controlled studies. Patients in the Brintellix® 20 mg group were receiving the starting dose of 10 mg before day 8 and were up-titrated to Brintellix® 20 mg on day 8.1
*p<0.05, **p<0.01, ***p<0.001 vs placebo;§ p<0.05 for Brintellix® 20 mg/day vs Brintellix®10 mg/day
The highest response rates at all times were seen with Brintellix® 20 mg, with a statistically significant difference versus 10 mg seen at Week 4 and 8.1 Brintellix® 20 mg was superior to placebo at all timepoints, while Brintellix® 10mg was superior to placebo at Week 6 and 8.1 An additional analysis confirmed that patients treated with Brintellix® 20mg had a significantly shorter time to symptomatic response compared to Brintellix® 10 mg or placebo.1
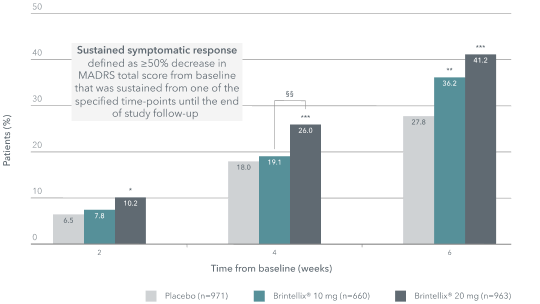
Brintellix® 20 mg provided an earlier sustained symptomatic response compared to 10 mg‡1
Sustained symptomatic response was defined as ≥50% decrease in MADRS total score from baseline that was sustained from one of the specified time-points until the end of study follow-up.1 At Week 2 (i.e. one week after dose up-titration to 20 mg), 10.2% of patients treated with Brintellix® 20 mg achieved sustained response, which was statistically significant to the 6.5% of patients treated with placebo. After one month treated with Brintellix®, significantly more patients treated with 20 mg treated sustained response as compared to those treated with 10 mg (26.0% vs 19.1%, p<0.01).
With Brintellix® 20 mg, significantly more patients achieved a general sustained symptomatic response# compared to patients treated with Brintellix® 10 mg over 8 weeks of treatment (36.0% versus 29.0%; p<0.05; OR, 1.33; p=0.01).1
Proportion of patients achieving sustained symptomatic response1
Adapted from Christensen MC et al. 2023.
‡Meta-analysis of six 8-week, fixed-dose, placebo-controlled studies. Patients in the Brintellix® 20 mg group were receiving the starting dose of 10 mg before day 8 and were up-titrated to Brintellix® 20 mg on day 8.1
*p<0.05, **p<0.01, ***p<0.001 vs placebo;§ p<0.05 for Brintellix® 20 mg/day vs Brintellix®10 mg/day
The highest response rates at all times were seen with Brintellix® 20 mg, with a statistically significant difference versus 10 mg seen at Week 4 and 8.1 Brintellix® 20 mg was superior to placebo at all timepoints, while Brintellix® 10mg was superior to placebo at Week 6 and 8.1 An additional analysis confirmed that patients treated with Brintellix® 20mg had a significantly shorter time to symptomatic response compared to Brintellix® 10 mg or placebo.1
Brintellix® 20 mg provided an earlier sustained symptomatic response compared to 10 mg‡1
Sustained symptomatic response was defined as ≥50% decrease in MADRS total score from baseline that was sustained from one of the specified time-points until the end of study follow-up.1 At Week 2 (i.e. one week after dose up-titration to 20 mg), 10.2% of patients treated with Brintellix® 20 mg achieved sustained response, which was statistically significant to the 6.5% of patients treated with placebo. After one month treated with Brintellix®, significantly more patients treated with 20 mg treated sustained response as compared to those treated with 10 mg (26.0% vs 19.1%, p<0.01).
With Brintellix® 20 mg, significantly more patients achieved a general sustained symptomatic response# compared to patients treated with Brintellix® 10 mg over 8 weeks of treatment (36.0% versus 29.0%; p<0.05; OR, 1.33; p=0.01).1
Proportion of patients achieving sustained symptomatic response1
Adapted from Christensen MC et al. 2023.
*p<0.05, **p<0.01, ***p<0.001 vs placebo;§§ p<0.01 for Brintellix® 20 mg vs Brintellix® 10 mg.
Depending on the individual patient response, the dose can be increased to a maximum of 20 mg/day or decreased to a minimum of 5 mg/day.3
Explore the incidence rates of the most frequent treatment-emergent adverse events (TEAEs) with Brintellix® 20 mg vs 10 mg.
Adapted from Christensen MC et al. 2023.
*p<0.05, **p<0.01, ***p<0.001 vs placebo;§§ p<0.01 for Brintellix® 20 mg vs Brintellix® 10 mg.
Depending on the individual patient response, the dose can be increased to a maximum of 20 mg/day or decreased to a minimum of 5 mg/day.3
Explore the incidence rates of the most frequent treatment-emergent adverse events (TEAEs) with Brintellix® 20 mg vs 10 mg.

† A study investigating the time characteristics of recovery in patients with MDD (N=2,848) found that >70% of patients who showed sustained early improvement (improvement defined as a 20% sustained baseline score reduction in HAM-D) within the first 2 weeks of treatment became sustained responders (response defined as a 50% sustained baseline score reduction in HAM-D) by Week 6. Treatments included in this study: imipramine, moclobemide, amitriptyline, fluoxetine, oxaprotiline, mirtazapine, paroxetine.2
‡ In a meta-analysis of pooled data in patients with MDD (n=2,620). Patients in the Brintellix® 20 mg group were receiving the starting dose of 10 mg during Week 1, followed by an up-titration to 20 mg on day 8.1
# General sustained response was defined as ≥50% decrease in MADRS total score from any time point in
addition to the last observed time point.1
Abbreviations:
HAM-D, Hamilton depression rating scale; HR, hazard ratio; MADRS, Montgomery-Åsberg depression rating
scale; MDD, major depressive disorder; OR, odds ratio; TEAE, treatment-emergent adverse event.
† A study investigating the time characteristics of recovery in patients with MDD (N=2,848) found that >70% of patients who showed sustained early improvement (improvement defined as a 20% sustained baseline score reduction in HAM-D) within the first 2 weeks of treatment became sustained responders (response defined as a 50% sustained baseline score reduction in HAM-D) by Week 6. Treatments included in this study: imipramine, moclobemide, amitriptyline, fluoxetine, oxaprotiline, mirtazapine, paroxetine.2
‡ In a meta-analysis of pooled data in patients with MDD (n=2,620). Patients in the Brintellix® 20 mg group were receiving the starting dose of 10 mg during Week 1, followed by an up-titration to 20 mg on day 8.1
# General sustained response was defined as ≥50% decrease in MADRS total score from any time point in
addition to the last observed time point.1
Abbreviations:
HAM-D, Hamilton depression rating scale; HR, hazard ratio; MADRS, Montgomery-Åsberg depression rating
scale; MDD, major depressive disorder; OR, odds ratio; TEAE, treatment-emergent adverse event.

