Brintellix® reduced emotional blunting in patients with MDD1
Brintellix® (vortioxetine) effectively reduced emotional blunting* in patients with major depressive disorder (MDD) with a partial response to their previous SSRIs/SNRIs.†1
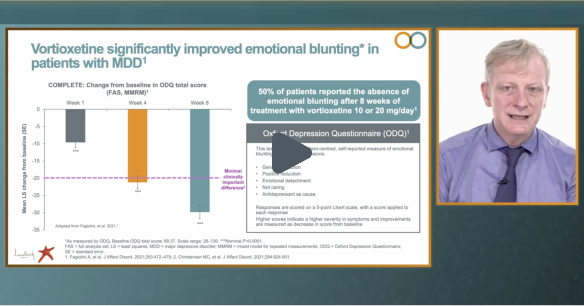
Watch the video of Professor Andrea Fagiolini (Division of Psychiatry at the University of Siena School of Medicine, Italy), the principal investigator of the COMPLETE study, presenting the main outcomes, including a significant improvement in emotional blunting in patients with MDD.‡1 COMPLETE is an interventional, 8-week, open-label, flexible-dose (10-20 mg/day) study of Brintellix® on emotional blunting in patients with MDD with a partial response to previous SSRIs/SNRIs†.1
Watch the video of Professor Andrea Fagiolini (Division of Psychiatry at the University of Siena School of Medicine, Italy), the principal investigator of the COMPLETE study, presenting the main outcomes, including a significant improvement in emotional blunting in patients with MDD.‡1 COMPLETE is an interventional, 8-week, open-label, flexible-dose (10-20 mg/day) study of Brintellix® on emotional blunting in patients with MDD with a partial response to previous SSRIs/SNRIs†.1

Patients with less emotional blunting (as measured by ODQ) displayed more energy and motivation (as measured by MEI) and greater overall functioning (as measured by SDS).1
The study confirmed the generally well-tolerated profile of Brintellix® as demonstrated in previous studies.3 The most common treatment-emergent adverse events (TEAEs; reported by >5% of the study group) were nausea (20.7%), headache (8.0%), dizziness (6.7%), vomiting (6.7%) and diarrhoea (6.0%).1
Patients with less emotional blunting (as measured by ODQ) displayed more energy and motivation (as measured by MEI) and greater overall functioning (as measured by SDS).1
The study confirmed the generally well-tolerated profile of Brintellix® as demonstrated in previous studies.3 The most common treatment-emergent adverse events (TEAEs; reported by >5% of the study group) were nausea (20.7%), headache (8.0%), dizziness (6.7%), vomiting (6.7%) and diarrhoea (6.0%).1
* As measured by ODQ, an effective self-report measure of the symptoms of emotional blunting in patients with depression.3
† Partial response defined by the investigators’ clinical judgement of symptom severity and type to an SSRI or SNRI monotherapy at approved doses for at least 6 weeks before the screening visit. Previous SSRIs included escitalopram, paroxetine, sertraline and citalopram; previous SNRIs included venlafaxine and duloxetine.1
‡ Emotional blunting was assessed using the ODQ total score; week 1 = -9.6, week 4 = -21.2, week 8 = -29.8. Depressive symptoms were assessed using the MADRS total score; at week 1 = -3.3, week 4 = -9.2, week 8 = 13.8. p<0.0001 for all changes vs baseline.1
¶ Patients with MDD were asked the gold standard standardised screening question on emotional blunting (yes/no) developed by Price J et al. 2012 at week 8. Emotional effects vary, but may include, for example, feeling emotionally numbed’ or blunted’ in some way; lacking positive emotions or negative emotions; feeling detached from the world around you; or just not caring’ about things that you used to care about. Have you experienced such emotional effects during the last 6 weeks?”. At baseline, all patients had to
report “Yes to this screening question.1
# As measured by the patient-reported ODQ. The suggested minimal clinically important difference for change in ODQ-26 scores is 20 points after 8 weeks of antidepressant treatment.2 With Brintellix® 10-20 mg, at week 8 a decrease of -29.8 points was achieved.1
¥ Change from baseline to week 8 in SDS total score (-7.7), work/school (-3.2), family life/home responsibilities (-2.5), social life (-2.4).1
§ Change from baseline to week 8 in MEI total score correlated with change from baseline in ODQ total score (-0.74, p<0.0001) and change from baseline in SDS total score (-0.67, p<0.0001).2
Abbreviations:
MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; MEI, motivation and energy inventory; ODQ, Oxford depression questionnaire; SDS, Sheehan disability scale; SNRI, Serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor
* As measured by ODQ, an effective self-report measure of the symptoms of emotional blunting in patients with depression.3
† Partial response defined by the investigators’ clinical judgement of symptom severity and type to an SSRI or SNRI monotherapy at approved doses for at least 6 weeks before the screening visit. Previous SSRIs included escitalopram, paroxetine, sertraline and citalopram; previous SNRIs included venlafaxine and duloxetine.1
‡ Emotional blunting was assessed using the ODQ total score; week 1 = -9.6, week 4 = -21.2, week 8 = -29.8. Depressive symptoms were assessed using the MADRS total score; at week 1 = -3.3, week 4 = -9.2, week 8 = 13.8. p<0.0001 for all changes vs baseline.1
¶ Patients with MDD were asked the gold standard standardised screening question on emotional blunting (yes/no) developed by Price J et al. 2012 at week 8. Emotional effects vary, but may include, for example, feeling emotionally numbed’ or blunted’ in some way; lacking positive emotions or negative emotions; feeling detached from the world around you; or just not caring’ about things that you used to care about. Have you experienced such emotional effects during the last 6 weeks?”. At baseline, all patients had to
report “Yes to this screening question.1
# As measured by the patient-reported ODQ. The suggested minimal clinically important difference for change in ODQ-26 scores is 20 points after 8 weeks of antidepressant treatment.2 With Brintellix® 10-20 mg, at week 8 a decrease of -29.8 points was achieved.1
¥ Change from baseline to week 8 in SDS total score (-7.7), work/school (-3.2), family life/home responsibilities (-2.5), social life (-2.4).1
§ Change from baseline to week 8 in MEI total score correlated with change from baseline in ODQ total score (-0.74, p<0.0001) and change from baseline in SDS total score (-0.67, p<0.0001).2
Abbreviations:
MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; MEI, motivation and energy inventory; ODQ, Oxford depression questionnaire; SDS, Sheehan disability scale; SNRI, Serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor

