Brintellix® may be increased up to 20 mg without compromising patients' tolerability1
Brintellix® (vortioxetine) is generally well tolerated across the therapeutic dose range (5-20 mg).1 In a meta-analysis, no clinically relevant increase in the incidence of treatment emergent adverse events (TEAEs) was not increased in patients who received Brintellix® 20 mg/day versus 10 mg/day.†1
Most frequent TEAEs with Brintellix® (≥4%; Days 8–56)‡1
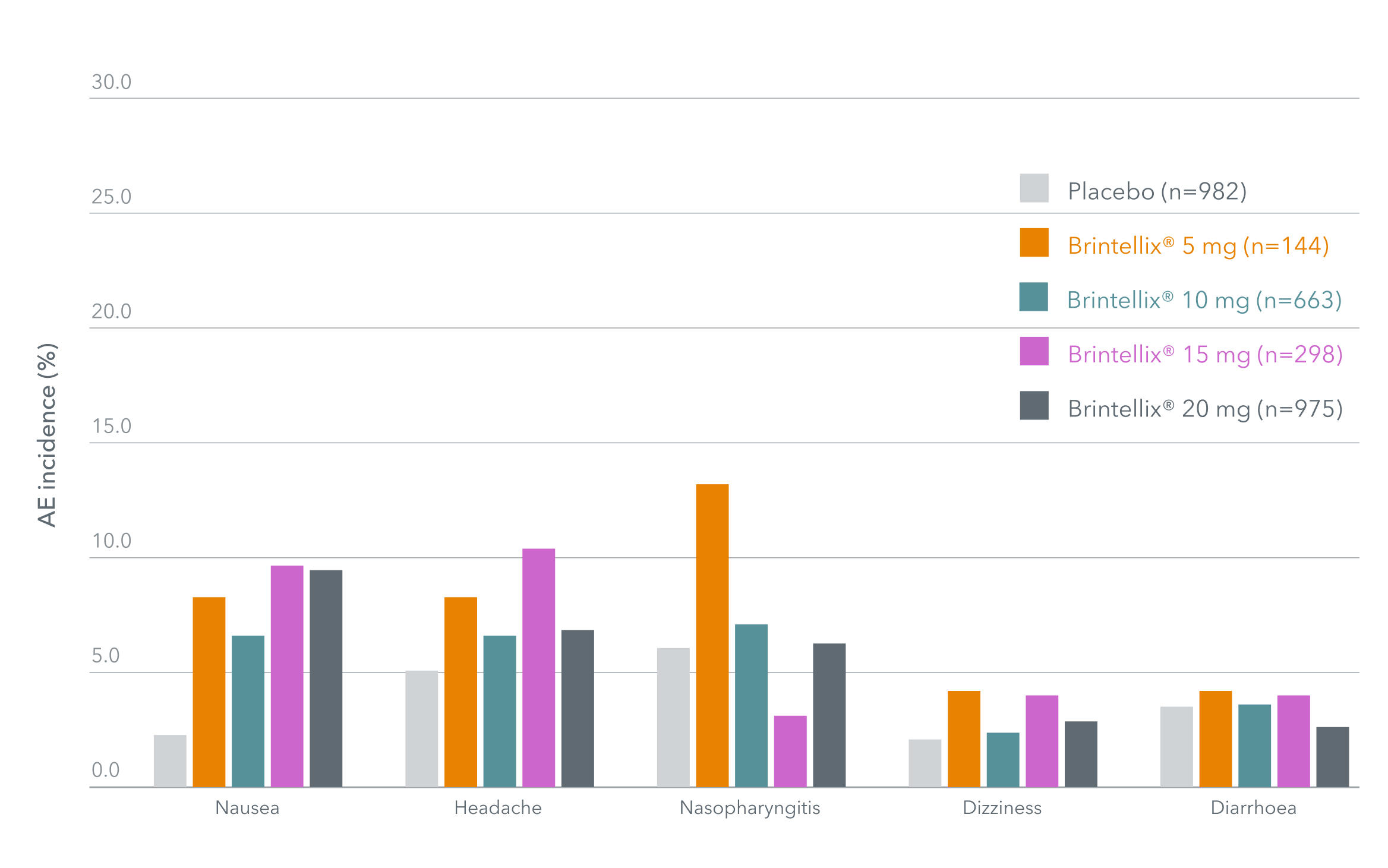
Adapted from Christensen MC, et al. 2023.
‡Only TEAEs with an incidence of ≥4% occurring in at least one treatment group are presented. In these fixed-dose studies, patients who were assigned to receive vortioxetine 20 mg received a 10 mg dose for the first week of the 8-week study. Data based on a meta-analysis of six short-term, fixed-dose, placebo-controlled studies.1
Raising the dose of Brintellix® from the recommended starting dose of 10 mg to 20 mg after Week 1 with the aim of improving efficacy did not compromise patients tolerability.†1,2
Adapted from Christensen MC, et al. 2023.
‡Only TEAEs with an incidence of ≥4% occurring in at least one treatment group are presented. In these fixed-dose studies, patients who were assigned to receive vortioxetine 20 mg received a 10 mg dose for the first week of the 8-week study. Data based on a meta-analysis of six short-term, fixed-dose, placebo-controlled studies.1
Raising the dose of Brintellix® from the recommended starting dose of 10 mg to 20 mg after Week 1 with the aim of improving efficacy did not compromise patients tolerability.†1,2

† Depending on individual patient response, the dose can be increased to a maximum of Brintellix® 20 mg/day or decreased to a minimum of 5 mg/day.2 For patients aged 65 years or older, a starting dose of 5 mg should be used.2
‡ Only TEAEs with an incidence of ≥4% occurring in at least one treatment group are presented. In these fixed-dose studies, patients who were assigned to receive Brintellix® 20 mg received a 10 mg dose for the first week of the 8-week study.1
Abbreviations: MDD, major depressive disorder; TEAE, treatment-emergent adverse event
† Depending on individual patient response, the dose can be increased to a maximum of Brintellix® 20 mg/day or decreased to a minimum of 5 mg/day.2 For patients aged 65 years or older, a starting dose of 5 mg should be used.2
‡ Only TEAEs with an incidence of ≥4% occurring in at least one treatment group are presented. In these fixed-dose studies, patients who were assigned to receive Brintellix® 20 mg received a 10 mg dose for the first week of the 8-week study.1
Abbreviations: MDD, major depressive disorder; TEAE, treatment-emergent adverse event

