Brintellix® may be increased to 20 mg after Week 1 without compromising tolerability1
Brintellix® (vortioxetine) may be up-titrated to 20 mg/day after Week 1 without compromising tolerability.1 No clinically relevant increase in treatment-emergent adverse events (TEAEs) was observed in patients with major depressive disorder (MDD) during the first week of treatment with Brintellix® 20 mg following up-titration (days 8-15).1
Rates of TEAEs‡ from baseline to Day 15#1
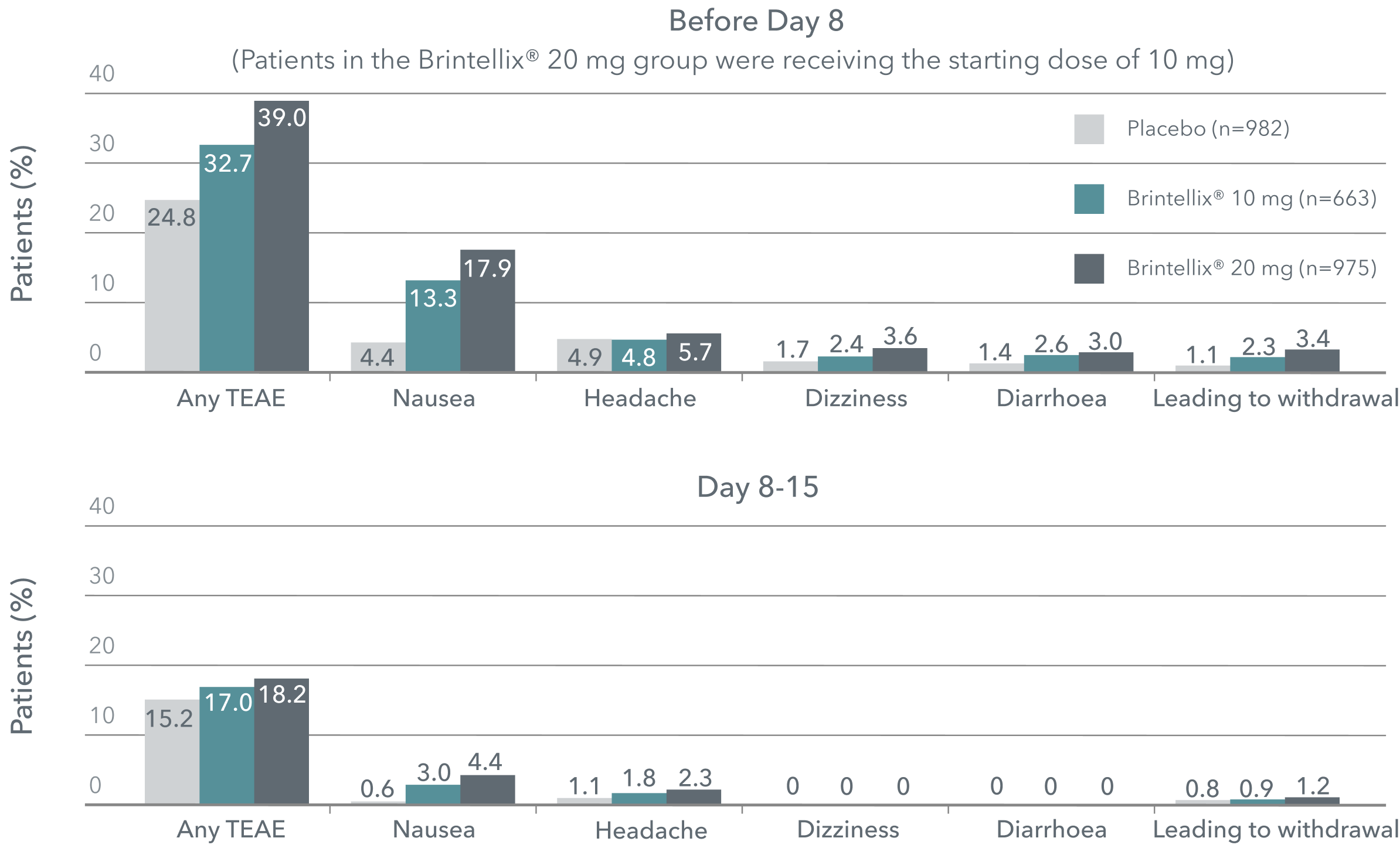
Adapted from Christensen MC et al. 2023.
‡ TEAEs by Medical Dictionary for Regulatory Activities preferred terms with incidence ≥2% in any group during either period.
# Meta-analysis of six 8-week, fixed-dose, placebo-controlled studies. Patients in the Brintellix® 20 mg group were receiving the starting dose of 10 mg before day 8 and were up-titrated to Brintellix® 20 mg on day 8.1
Brintellix® is generally well tolerated across the therapeutic dose range (5-20 mg).1,2 Irrespective of Brintellix® dosage, the most commonly reported TEAEs were nausea and headache.1
Adapted from Christensen MC et al. 2023.
‡ TEAEs by Medical Dictionary for Regulatory Activities preferred terms with incidence ≥2% in any group during either period.
# Meta-analysis of six 8-week, fixed-dose, placebo-controlled studies. Patients in the Brintellix® 20 mg group were receiving the starting dose of 10 mg before day 8 and were up-titrated to Brintellix® 20 mg on day 8.1
Brintellix® is generally well tolerated across the therapeutic dose range (5-20 mg).1,2 Irrespective of Brintellix® dosage, the most commonly reported TEAEs were nausea and headache.1


Abbreviations:
MDD, major depressive disorder; TEAE, treatment-emergent adverse event.
Abbreviations:
MDD, major depressive disorder; TEAE, treatment-emergent adverse event.

