Brintellix® improves overall functioning with the greatest improvement observed at 20mg1
39% of patients with major depressive disorder (MDD) exhibit significant functional impairment†2 , which is predictive of subsequent relapse3,4 and can persist even when other symptoms resolve.4-6 Restoring patients’ function is a key treatment goal in MDD.7-11
Brintellix® (vortioxetine) significantly improves overall functioning at work, at home and socially in patients with MDD.‡1
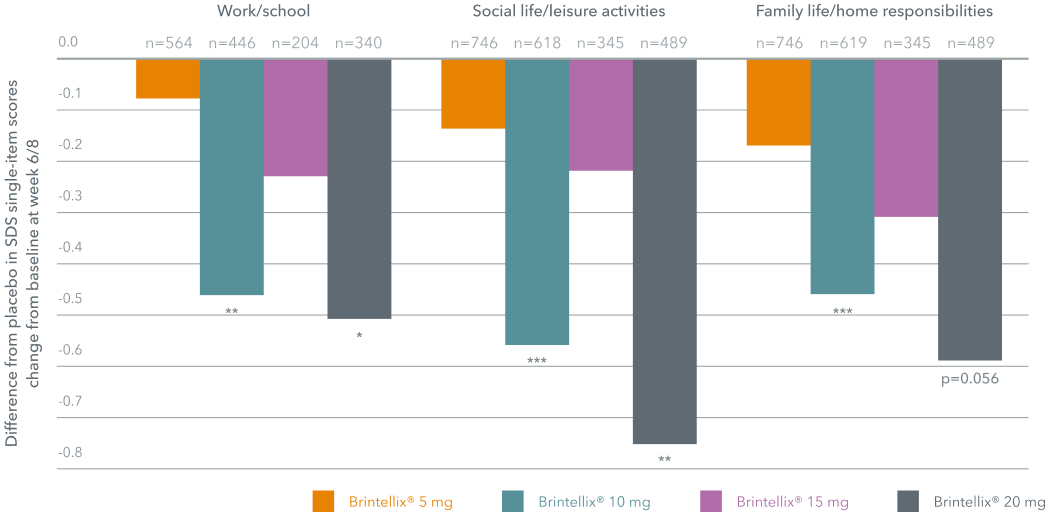
In a meta-analysis, Brintellix® (5-20 mg) treatment for 6 or 8 weeks improved overall functioning as well as functional remission§ in adults with MDD, with a dose response relationship.‡1
Change in SDS single-item scores from baseline‡1
Adapted from Florea I et al. 2017
*p<0.05; **p<0.01; ***p<0.001 vs placebo.
‡ Brintellix® 5 mg and 15 mg doses were not shown to be statistically significant.1 Brintellix® 5 mg is the recommended starting dose in elderly patients only.7
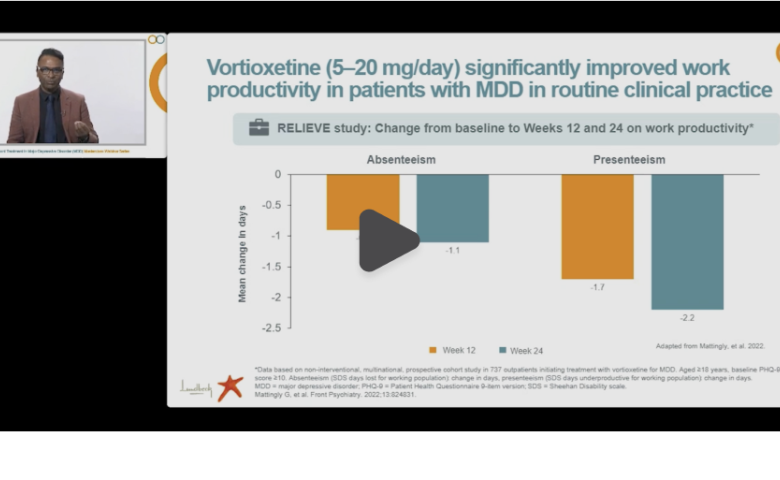
Watch the video with Dr. Pratap Chokka, Clinical Professor of Psychiatry at the University of Alberta (Canada), discussing how Brintellix®, in a dose-dependent manner, helps MDD patients achieve treatment goals by improving functional outcomes in their daily life.1
Dr Chokka also discusses further study outcomes, where Brintellix® (5-20 mg/day) showed significant improvements in symptoms of depression (PHQ-9), cognitive symptoms and performance (PDQ-D-5 and DSST) and health related quality of life (EQ-5D-5L) scores at Week 24.#1
Adapted from Florea I et al. 2017
*p<0.05; **p<0.01; ***p<0.001 vs placebo.
‡ Brintellix® 5 mg and 15 mg doses were not shown to be statistically significant.1 Brintellix® 5 mg is the recommended starting dose in elderly patients only.7
Watch the video with Dr. Pratap Chokka, Clinical Professor of Psychiatry at the University of Alberta (Canada), discussing how Brintellix®, in a dose-dependent manner, helps MDD patients achieve treatment goals by improving functional outcomes in their daily life.1
Dr Chokka also discusses further study outcomes, where Brintellix® (5-20 mg/day) showed significant improvements in symptoms of depression (PHQ-9), cognitive symptoms and performance (PDQ-D-5 and DSST) and health related quality of life (EQ-5D-5L) scores at Week 24.#1

† Functional impairment is defined as having an SDS total score >6.2
‡ Data based on a meta-analysis of nine short-term, randomised, placebo-controlled, fixed-dose clinical studies of Brintellix®
in adult patients with MDD, with the objective to assess the effect of Brintellix® on overall functioning using the Sheehan Disability Scale (SDS) in adults with MDD. Patients treated with Brintellix® 10 mg and 20 mg significantly improved overall functioning as measured by SDS, compared with placebo at 8 weeks. To account for previously identified issues related to the SDS work score, sensitivity analyses were conducted in which scores were calculated using three methods: (A) CRF worst case, where work related information were used to impute missing work scores, (B) imputed average scores, where the average of the two available items – social and family – is used to impute the missing work items, (C) assumed worst case score, where a worst case score of 10 is assumed and imputed for all missing work items.1
§ Functional remission was defined as an SDS total score of ≤6.1
# Data based on non-interventional, multinational, prospective cohort study in 737 outpatients initiating treatment with Brintellix® for MDD.13
Abbreviations:
DSST, digital symbol substitution test; EQ-5D-5L, EuroQol 5 dimensions 5 levels; MDD, major depressive disorder; PHQ-9, 9-question patient health questionnaire; PHQ-D-5, 5-item perceived deficits questionnaire – depression; SDS, Sheehan disability score.
† Functional impairment is defined as having an SDS total score >6.2
‡ Data based on a meta-analysis of nine short-term, randomised, placebo-controlled, fixed-dose clinical studies of Brintellix®
in adult patients with MDD, with the objective to assess the effect of Brintellix® on overall functioning using the Sheehan Disability Scale (SDS) in adults with MDD. Patients treated with Brintellix® 10 mg and 20 mg significantly improved overall functioning as measured by SDS, compared with placebo at 8 weeks. To account for previously identified issues related to the SDS work score, sensitivity analyses were conducted in which scores were calculated using three methods: (A) CRF worst case, where work related information were used to impute missing work scores, (B) imputed average scores, where the average of the two available items – social and family – is used to impute the missing work items, (C) assumed worst case score, where a worst case score of 10 is assumed and imputed for all missing work items.1
§ Functional remission was defined as an SDS total score of ≤6.1
# Data based on non-interventional, multinational, prospective cohort study in 737 outpatients initiating treatment with Brintellix® for MDD.13
Abbreviations:
DSST, digital symbol substitution test; EQ-5D-5L, EuroQol 5 dimensions 5 levels; MDD, major depressive disorder; PHQ-9, 9-question patient health questionnaire; PHQ-D-5, 5-item perceived deficits questionnaire – depression; SDS, Sheehan disability score.