Brintellix® improved motivation and energy in patients with MDD1
Improvements in motivation and energy (as measured by the MEI, motivation and energy inventory) in patients with major depressive disorder (MDD) treated with Brintellix® (vortioxetine) 10-20 mg/day were not only statistically significant, but also above clinically meaningful thresholds.†1,2
Beyond symptom resolution, patients with MDD report a broad range of treatment goals such as generalised goals, e.g. “feeling better”, or specific physical health goals.3 A recent self-completed survey revealed that over 80% of patients with MDD reported low motivation and lack of energy (fatigue) in the acute phase of MDD, and about 70% kept reporting these symptoms in the remission phase.#4
Watch the video of Professor Pratap Chokka (Division of Psychiatry at the University of Alberta, Canada), presenting the main outcomes of the COMPLETE study, an interventional, 8-week, open-label, flexible-dose (10-20 mg) study of Brintellix® on emotional blunting in patients with MDD with a partial response to previous SSRIs/SNRIs§.1 The COMPLETE study explored the effectiveness of Brintellix® (vortioxetine) 10-20 mg/day on motivation and energy (measured with the motivation and energy inventory, MEI, secondary endpoint).1
With Brintellix® 10-20 mg/day, significant and clinically meaningful improvements were seen across all MEI subdomains, including physical energy, cognitive energy and social motivation, at week 8.1
Change in MEI scores from baselines (FAS, MMRM)
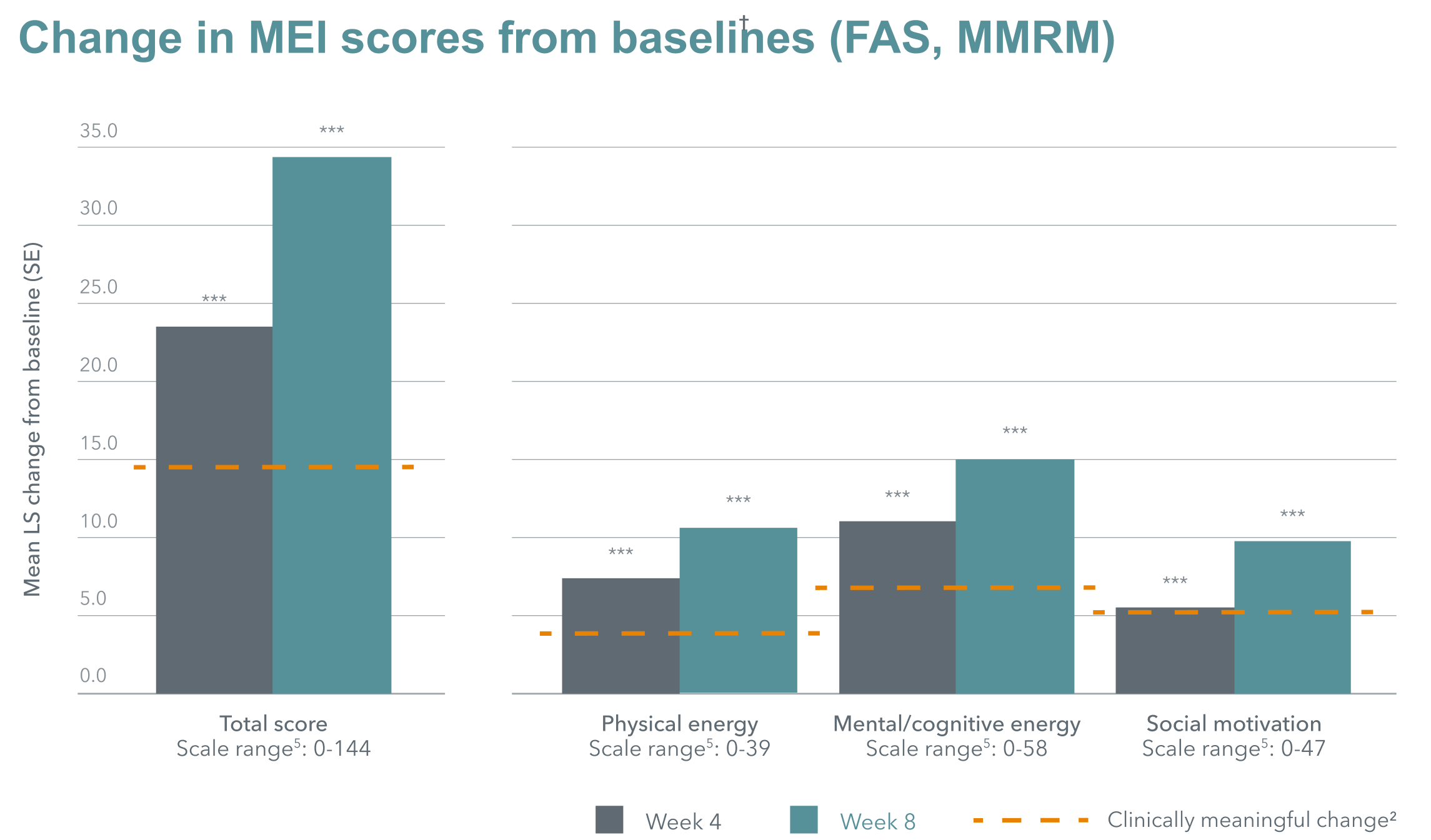
Adapted from: Fagiolini A et al. 2021.
Baseline MEI total score = 44.1
***nominal p<0.0001.
Adapted from: Fagiolini A et al. 2021.
Baseline MEI total score = 44.1
***nominal p<0.0001.

The study confirmed the generally well-tolerated profile of Brintellix® as demonstrated in previous studies.6 The most common treatment-emergent adverse events (TEAEs; reported by >5% of the study group) were nausea (20.7%), headache (8.0%), dizziness (6.7%), vomiting (6.7%) and diarrhoea (6.0%).1
The study confirmed the generally well-tolerated profile of Brintellix® as demonstrated in previous studies.6 The most common treatment-emergent adverse events (TEAEs; reported by >5% of the study group) were nausea (20.7%), headache (8.0%), dizziness (6.7%), vomiting (6.7%) and diarrhoea (6.0%).1
† Improvements of ≥14.7 points in MEI total score are considered clinically meaningful.2
# Data based on a self-completed online survey in patients with MDD who are using a prescribed antidepressant (n=752); 84% of patients reported fatigue or lack of energy, 85% of patients reported low motivation during the acute phase (n=300) and 70% and 68% during remission, respectively.3
§ Partial response defined by the investigators’ clinical judgement of symptom severity and type to an SSRI or SNRI monotherapy at approved doses for at least 6 weeks before the screening visit. Previous SSRIs included escitalopram, paroxetine, sertraline and citalopram; previous SNRIs included venlafaxine and duloxetine.1
¶ As measured by ODQ (emotional blunting), MADRS (depressive symptoms), DSST (cognitive performance) and SDS (overall functioning). Change from baseline in MEI total score significantly correlated with change from baseline in ODQ total score (-0.74, p<0.0001), change from baseline in MADRS total score (-0.58, p<0.0001), change from baseline in DSST total score (0.26, p=0.0024) and change from baseline in SDS total score (-0.67, p<0.0001).2
Abbreviations:
DSST, digit symbol substitution test; FAS, full analysis set; MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; MEI; motivation and energy inventory; MMRM, mixed model for repeated measurements; ODQ, Oxford depression questionnaire; SDS, Sheehan disability scale; SNRI; serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
† Improvements of ≥14.7 points in MEI total score are considered clinically meaningful.2
# Data based on a self-completed online survey in patients with MDD who are using a prescribed antidepressant (n=752); 84% of patients reported fatigue or lack of energy, 85% of patients reported low motivation during the acute phase (n=300) and 70% and 68% during remission, respectively.3
§ Partial response defined by the investigators’ clinical judgement of symptom severity and type to an SSRI or SNRI monotherapy at approved doses for at least 6 weeks before the screening visit. Previous SSRIs included escitalopram, paroxetine, sertraline and citalopram; previous SNRIs included venlafaxine and duloxetine.1
¶ As measured by ODQ (emotional blunting), MADRS (depressive symptoms), DSST (cognitive performance) and SDS (overall functioning). Change from baseline in MEI total score significantly correlated with change from baseline in ODQ total score (-0.74, p<0.0001), change from baseline in MADRS total score (-0.58, p<0.0001), change from baseline in DSST total score (0.26, p=0.0024) and change from baseline in SDS total score (-0.67, p<0.0001).2
Abbreviations:
DSST, digit symbol substitution test; FAS, full analysis set; MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; MEI; motivation and energy inventory; MMRM, mixed model for repeated measurements; ODQ, Oxford depression questionnaire; SDS, Sheehan disability scale; SNRI; serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.


