Brintellix® efficacy increases with increasing dose†1,2
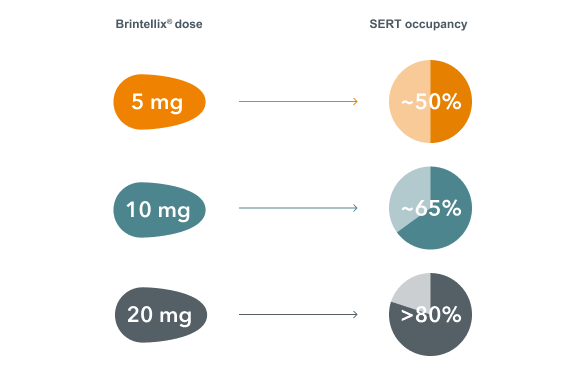
Brintellix® (vortioxetine) has a multi-modal mechanism of action (MoA) and is specifically designed to inhibit the serotonin transporter (SERT) and modulate serotonin receptor activity.2 This leads to modulation of neurotransmission across several systems, predominantly the serotonin, but also the norepinephrine, dopamine, histamine, acetylcholine, GABA and glutamate systems.2 However, the SERT occupancy depends on Brintellix® dose, ranging between ~50% to >80%.3
Brintellix® binds to SERT and serotonin receptors at clinically relevant doses, as shown in the graph below.3,4
Target occupancies with Brintellix®†3,4
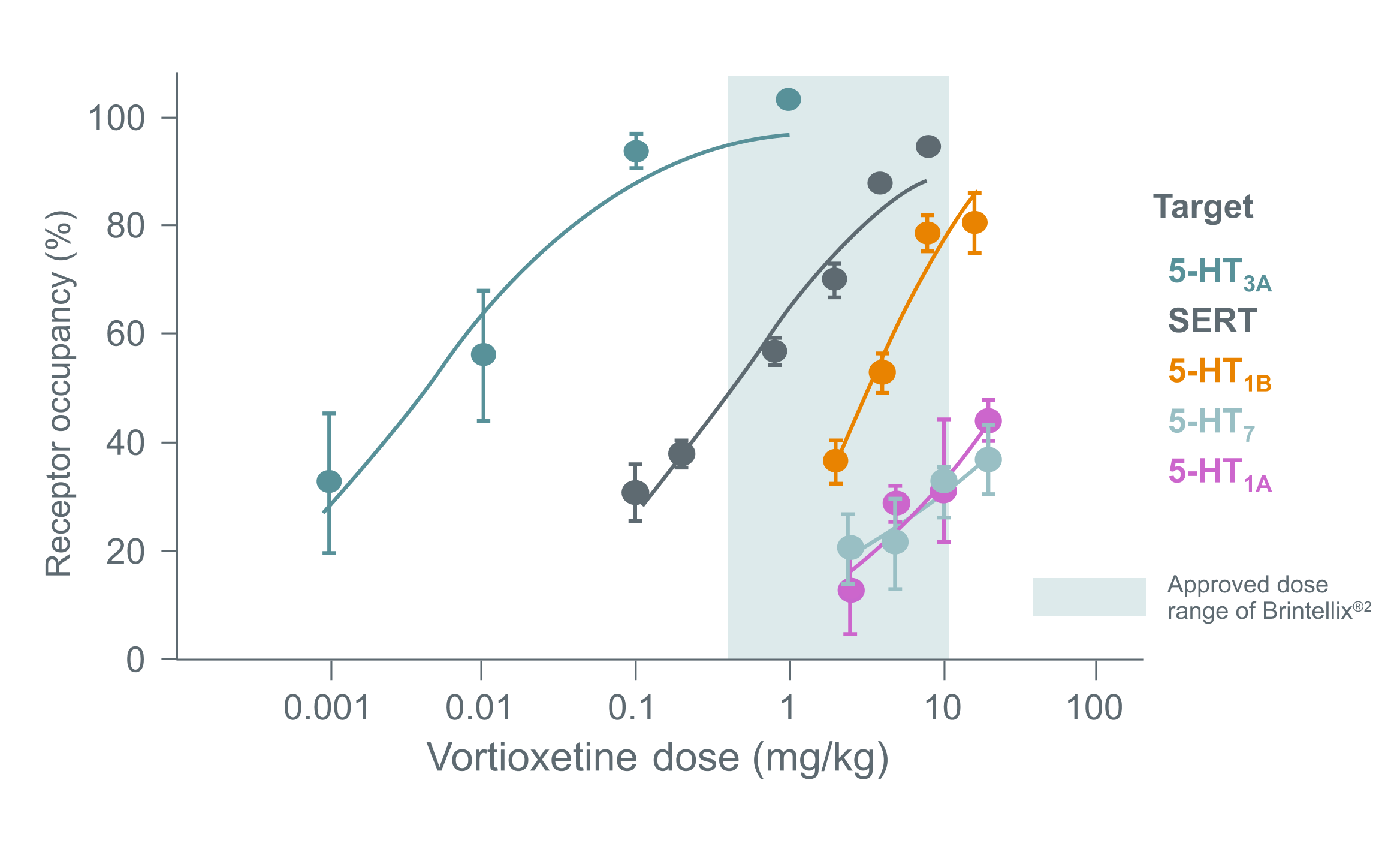
Adapted from Sanchez C et al. 2015.
† Graph shows the multi-target occupancies (% of total binding) of Brintellix® in rats 1 hour after subcutaneous dosing with Brintellix®. The approved dose range of Brintellix® (5-20 mg) spans ~50% to >80% SERT occupancy. 5-HT1A and 5-HT7 receptor affinities in rats are >10-fold less than in humans, and it therefore appears reasonable to conclude that all Brintellix®’s targets are likely to be occupied at clinically relevant doses, primarily 5-HT3 receptors and SERT at the lower doses and also 5-HT1B, 5-HT1A and 5-HT7 receptors at the higher doses.3
The precise contribution of the individual targets to the observed pharmacodynamic profile remains unclear and caution should be applied when extrapolating animal data directly.
Looking at the relationship between dose of Brintellix® and SERT occupancy, the clinically effective dose range of Brintellix® (5-20 mg) spans ~50% to >80% SERT occupancy.3 In order to achieve ~80% SERT occupancy, that corresponds to the approved dose ranges of many commonly used SSRIs and SNRIs‡5, a dose of 20 mg/day is required.3
A dose-related increase in SERT occupancy at steady-state conditions was observed in healthy individuals with Brintellix®3
Adapted from Sanchez C et al. 2015.
† Graph shows the multi-target occupancies (% of total binding) of Brintellix® in rats 1 hour after subcutaneous dosing with Brintellix®. The approved dose range of Brintellix® (5-20 mg) spans ~50% to >80% SERT occupancy. 5-HT1A and 5-HT7 receptor affinities in rats are >10-fold less than in humans, and it therefore appears reasonable to conclude that all Brintellix®’s targets are likely to be occupied at clinically relevant doses, primarily 5-HT3 receptors and SERT at the lower doses and also 5-HT1B, 5-HT1A and 5-HT7 receptors at the higher doses.3
The precise contribution of the individual targets to the observed pharmacodynamic profile remains unclear and caution should be applied when extrapolating animal data directly.
Looking at the relationship between dose of Brintellix® and SERT occupancy, the clinically effective dose range of Brintellix® (5-20 mg) spans ~50% to >80% SERT occupancy.3 In order to achieve ~80% SERT occupancy, that corresponds to the approved dose ranges of many commonly used SSRIs and SNRIs‡5, a dose of 20 mg/day is required.3
A dose-related increase in SERT occupancy at steady-state conditions was observed in healthy individuals with Brintellix®3
Considering the dose-response of Brintellix®, there is a potential efficacy advantage in increasing the dose up to 20 mg before considering switching medications, when there is a suboptimal initial response.3,4,6 The starting and recommended dose of 10 mg may be increased up to 20 mg or decreased to a minimum of 5 mg depending on patients’ individual response.2 For patients aged ≥ 65 years with MDD, the recommended starting dose is 5 mg.2
Brintellix® may be increased to 20 mg after Week 1 without compromising tolerability§7
Analysis of 11 short-term MDD studies showed that Brintellix® has a low rate of adverse events (AEs), even at higher doses.8 Unlike SSRIs¶9, a dose increase with Brintellix® is not associated with increased rates of AEs, meaning tolerability is not compromised.8
Adverse events with an incidence of ≥5% in any dose group during the core treatment period in 11 short-term MDD studies8
Considering the dose-response of Brintellix®, there is a potential efficacy advantage in increasing the dose up to 20 mg before considering switching medications, when there is a suboptimal initial response.3,4,6 The starting and recommended dose of 10 mg may be increased up to 20 mg or decreased to a minimum of 5 mg depending on patients’ individual response.2 For patients aged ≥ 65 years with MDD, the recommended starting dose is 5 mg.2
Brintellix® may be increased to 20 mg after Week 1 without compromising tolerability§7
Analysis of 11 short-term MDD studies showed that Brintellix® has a low rate of adverse events (AEs), even at higher doses.8 Unlike SSRIs¶9, a dose increase with Brintellix® is not associated with increased rates of AEs, meaning tolerability is not compromised.8
Adverse events with an incidence of ≥5% in any dose group during the core treatment period in 11 short-term MDD studies8
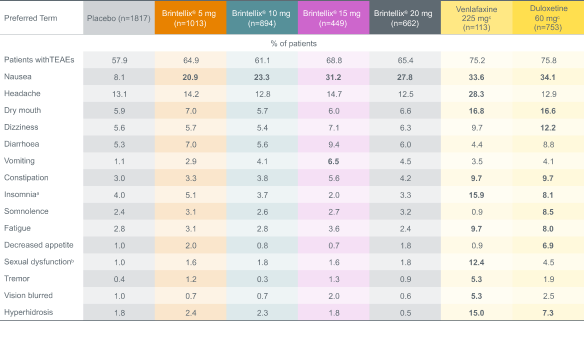
Adapted from: Baldwin DS et al. 2016a.
% values in bold are ≥5% and >2x placebo.
a Includes the preferred terms: insomnia, initial insomnia, middle insomnia, hyposomnia, sleep disorder, dyssomnia, poor quality sleep and terminal insomnia.
b Includes the preferred terms: libido decreased, ejaculation delayed, ejaculation disorder, orgasm abnormal, anorgasmia, disturbance in sexual arousal, ejaculation failure, erectile dysfunction, loss of libido, orgasmic sensation decreased, sexual dysfunction, and vulvovaginal dryness.
c Duloxetine and venlafaxine were included as active references for study validation, not for comparison of effect size.
Adapted from: Baldwin DS et al. 2016a.
% values in bold are ≥5% and >2x placebo.
a Includes the preferred terms: insomnia, initial insomnia, middle insomnia, hyposomnia, sleep disorder, dyssomnia, poor quality sleep and terminal insomnia.
b Includes the preferred terms: libido decreased, ejaculation delayed, ejaculation disorder, orgasm abnormal, anorgasmia, disturbance in sexual arousal, ejaculation failure, erectile dysfunction, loss of libido, orgasmic sensation decreased, sexual dysfunction, and vulvovaginal dryness.
c Duloxetine and venlafaxine were included as active references for study validation, not for comparison of effect size.

In this video Professor Andrea Fagiolini discusses how the dose response of Brintellix® improves efficacy without compromising tolerability for patients with MDD.
In this video Professor Andrea Fagiolini discusses how the dose response of Brintellix® improves efficacy without compromising tolerability for patients with MDD.

‡ Striatal 5-HTT binding potential measured in 77 subjects before and after 4 weeks of medication administration. Binding potential is proportional to the density of receptors not blocked by medication. Subjects received citalopram, fluoxetine, sertraline, paroxetine, or extended-release venlafaxine. Healthy subjects received subtherapeutic doses; subjects with mood and anxiety disorders received therapeutic doses.5
¶ SSRIs studied included citalopram, escitalopram, paroxetine and sertraline. As assessed in a systematic review and dose response meta-analysis of double-blind, randomised controlled trials that examined fixed doses of five SSRIs, venlafaxine, or mirtazapine in the acute treatment of adults with major depressive disorder. Doses of SSRIs were converted to fluoxetine equivalents.9
# Data based on a meta-analysis of six short-term, fixed-dose, placebo-controlled studies. In these fixed-dose studies, patients who were assigned to receive Brintellix® 20 mg received a 10 mg dose for the first week of the 8-week study. MDD symptoms were measured using MADRS single items at Week 8. Brintellix® 20 mg demonstrated statistically significant improvements vs. placebo on all MADRS single items.1
Abbreviations:
AE, adverse event; GABA, gamma-aminobutyric acid; MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; MoA, mechanism of action; SERT, serotonin reuptake transporter; SNRI, serotonin and norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors
‡ Striatal 5-HTT binding potential measured in 77 subjects before and after 4 weeks of medication administration. Binding potential is proportional to the density of receptors not blocked by medication. Subjects received citalopram, fluoxetine, sertraline, paroxetine, or extended-release venlafaxine. Healthy subjects received subtherapeutic doses; subjects with mood and anxiety disorders received therapeutic doses.5
¶ SSRIs studied included citalopram, escitalopram, paroxetine and sertraline. As assessed in a systematic review and dose response meta-analysis of double-blind, randomised controlled trials that examined fixed doses of five SSRIs, venlafaxine, or mirtazapine in the acute treatment of adults with major depressive disorder. Doses of SSRIs were converted to fluoxetine equivalents.9
# Data based on a meta-analysis of six short-term, fixed-dose, placebo-controlled studies. In these fixed-dose studies, patients who were assigned to receive Brintellix® 20 mg received a 10 mg dose for the first week of the 8-week study. MDD symptoms were measured using MADRS single items at Week 8. Brintellix® 20 mg demonstrated statistically significant improvements vs. placebo on all MADRS single items.1
Abbreviations:
AE, adverse event; GABA, gamma-aminobutyric acid; MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; MoA, mechanism of action; SERT, serotonin reuptake transporter; SNRI, serotonin and norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors