Brintellix® effectively improved overall functioning in patients with MDD with a partial response to previous SSRIs/ SNRI treatment
Inadequate treatment response and emotional blunting are common challenges with SSRI/SNRI for Major Depressive Disorder (MDD). Approximately 50% of all patients have a partial response to these therapies in terms of depressive-symptoms resolution.2 In addition, about 50-60% of all patients with MDD treated with SSRIs or SNRIs report some degree of emotional blunting, which may be related to the medication.3,4 Patients with MDD who experienced a partial response and emotional blunting after treatment with SSRI or SNRI, reported significant improvements in depressive symptoms, emotional blunting and overall functioning with Brintellix® (vortioxetine) 10-20 mg/day, over 8 Weeks of treatment.1
The COMPLETE study is an interventional, 8-Week, open-label, flexible-dose (10-20 mg/day) study of Brintellix® on emotional blunting (measured by the Oxford Depression Questionnaire, ODQ) and functional outcome (measured by self-rated
depression scale, SDS) in patients with MDD with a partial response to SSRI/ SNRI monotherapy at adequate dose for ≥6 Weeks‡1. From Week 1, Brintellix® significantly reduced all ODQ subdomains, and at Week 8 there were significant
improvements in overall functioning.1 See the key findings on functioning outcome from this study below.
COMPLETE: Change from baseline to Week 8 in SDS score (FAS,MMRM)
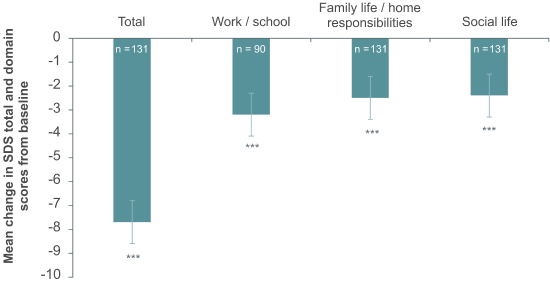
***nominal p<0.0001.
Significant improvements from baseline to Week 8 in overall functioning, as measured by the SDS, were observed for the total score as well as the single items, most pronouncedly in the work/school domain. The improvement in emotional blunting with Brintellix® treatment was strongly and significantly correlated with improvement in motivation, energy and overall functioning.1
In contrast, studies have shown that following SSRI treatment, MDD patients continue to suffer from functional impairments and reduced quality of life. For instance in the STAR*D trial, a large majority of MDD patients were observed to experience severe impairments in QoL and functioning pre-SSRI treatment as measured by the QLESQa (86% patients), the SF12-MCSa (71.2% patients), and
the WSASb (65.2% patients). Post-SSRI treatment, a substantial percentage ofpatients were observed to still suffer from both functional and QoL impairments5, with a follow-up observational study showing that only 52.9% of the patients achieved clinical remissionc, with functional remissiond
and recoverye in only 37.7% and 34.2% of the patients respectively6. See the key findings on functioning and QoL outcomes from this study below.
STAR*D: Percentage of patients reporting severe impairments in QoL and functioning scores pre- and post-SSRI treatment5
***nominal p<0.0001.
Significant improvements from baseline to Week 8 in overall functioning, as measured by the SDS, were observed for the total score as well as the single items, most pronouncedly in the work/school domain. The improvement in emotional blunting with Brintellix® treatment was strongly and significantly correlated with improvement in motivation, energy and overall functioning.1
In contrast, studies have shown that following SSRI treatment, MDD patients continue to suffer from functional impairments and reduced quality of life. For instance in the STAR*D trial, a large majority of MDD patients were observed to experience severe impairments in QoL and functioning pre-SSRI treatment as measured by the QLESQa (86% patients), the SF12-MCSa (71.2% patients), and
the WSASb (65.2% patients). Post-SSRI treatment, a substantial percentage ofpatients were observed to still suffer from both functional and QoL impairments5, with a follow-up observational study showing that only 52.9% of the patients achieved clinical remissionc, with functional remissiond
and recoverye in only 37.7% and 34.2% of the patients respectively6. See the key findings on functioning and QoL outcomes from this study below.
STAR*D: Percentage of patients reporting severe impairments in QoL and functioning scores pre- and post-SSRI treatment5
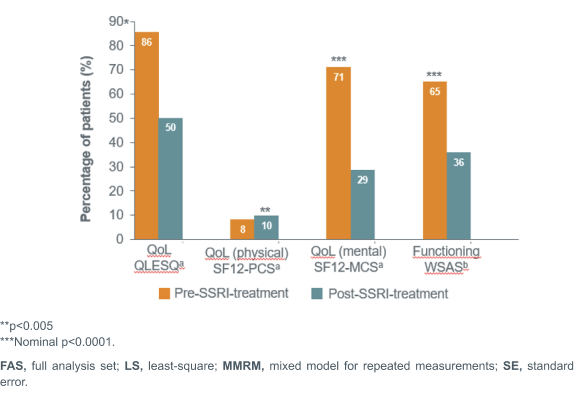
**p<0.005
***Nominal p<0.0001.
In summary from above, Brintellix® treatment improves functioning, whilst the use of SSRIs shows limited improvements in functioning and quality of life.
Want to learn more on the best way to switch from an SSRI/SNRI to Brintellix®?
Learn more from the clinical experiences of Prof Andrew Cutler and Prof Andrea Fagiolini‡
**p<0.005
***Nominal p<0.0001.
In summary from above, Brintellix® treatment improves functioning, whilst the use of SSRIs shows limited improvements in functioning and quality of life.
Want to learn more on the best way to switch from an SSRI/SNRI to Brintellix®?
Learn more from the clinical experiences of Prof Andrew Cutler and Prof Andrea Fagiolini‡
‡Partial response defined by the investigators clinical judgement of symptom severity and type to an SSRI or SNRI monotherapy at approved doses for at least 6 Weeks before the screening visit. Previous SSRIs included escitalopram, paroxetine, sertraline and citalopram; previous SNRIs included venlafaxine and duloxetine.1
a Severe QoL impairment measured by Q-LES-Q <55.7, SF-12 PCS <30, SF-12 MCS <30;
bSevere functioning impairment measured by WSAS >20;
cClinical remission was defined as a QIDS-SR16 score ≤5;
dFunctional remission was defined as having no or minimum disability on the SDS (score <3 on each of the
three subscales) and no days of reduced productivity in the previous Week6;
eRecovery was defined as having both clinical and functional remission
Abbreviations:
FAS, Fatigue Assessment Scale; MADRS, Montgomery-Åsberg depression rating scale; MDD, major
depressive disorder; MEI, motivation and energy inventory; MMRM, Mixed model for repeated
measurements; ODQ, Oxford depression questionnaire; QIDS-SR, Quick Inventory of Depressive
Symptomatology-Self-Report; QLESQ, Quality of Life measure: Quality of Life, Enjoyment, and satisfaction
questionnaire-short form; QoL, quality of life; SDS, Sheehan disability scale; SE, standard error; SF12-PCS,
Short Form Survey Physical Component Summary; SF12-MCS, SF12 Mental Component Summary; SNRI,
serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; STAR*D,
Sequenced Treatment Alternatives to Relieve Depression; WSAS, work and social adjustment scale.
‡Partial response defined by the investigators clinical judgement of symptom severity and type to an SSRI or SNRI monotherapy at approved doses for at least 6 Weeks before the screening visit. Previous SSRIs included escitalopram, paroxetine, sertraline and citalopram; previous SNRIs included venlafaxine and duloxetine.1
a Severe QoL impairment measured by Q-LES-Q <55.7, SF-12 PCS <30, SF-12 MCS <30;
bSevere functioning impairment measured by WSAS >20;
cClinical remission was defined as a QIDS-SR16 score ≤5;
dFunctional remission was defined as having no or minimum disability on the SDS (score <3 on each of the
three subscales) and no days of reduced productivity in the previous Week6;
eRecovery was defined as having both clinical and functional remission
Abbreviations:
FAS, Fatigue Assessment Scale; MADRS, Montgomery-Åsberg depression rating scale; MDD, major
depressive disorder; MEI, motivation and energy inventory; MMRM, Mixed model for repeated
measurements; ODQ, Oxford depression questionnaire; QIDS-SR, Quick Inventory of Depressive
Symptomatology-Self-Report; QLESQ, Quality of Life measure: Quality of Life, Enjoyment, and satisfaction
questionnaire-short form; QoL, quality of life; SDS, Sheehan disability scale; SE, standard error; SF12-PCS,
Short Form Survey Physical Component Summary; SF12-MCS, SF12 Mental Component Summary; SNRI,
serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; STAR*D,
Sequenced Treatment Alternatives to Relieve Depression; WSAS, work and social adjustment scale.


