Achieving higher rates of treatment success and satisfaction with Brintellix®1,2
Treatment adherence in patients with major depressive disorder (MDD) is strongly influenced by the patient’s individual treatment response, tolerability and general treatment satisfaction.3,4 Brintellix® (vortioxetine) demonstrated higher rates of treatment success versus the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine in patients with MDD†1, where treatment success was defined as a 50% reduction in MADRS total score in absence of treatment emergent adverse event (TEAEs).1
Successfully treated patients (STP) at week 8†1
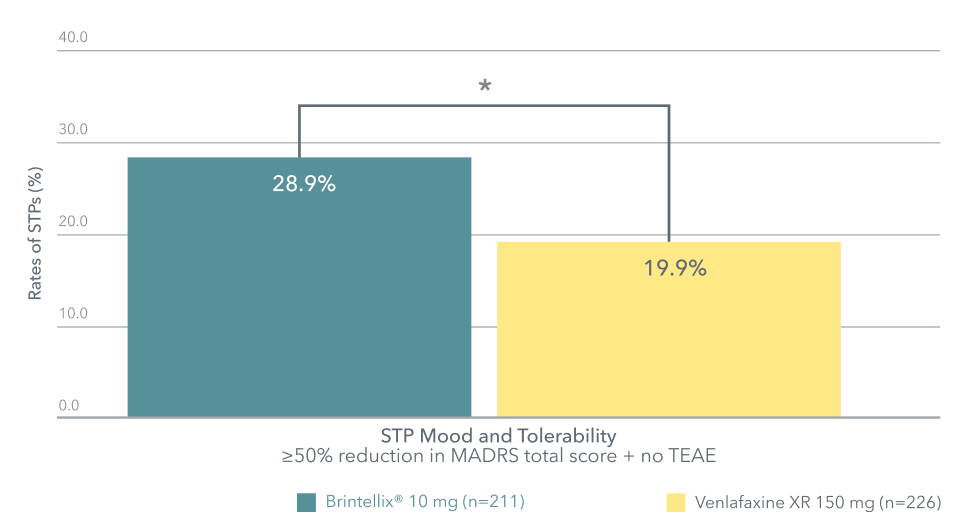
Adapted from Wang G et al. 2020
*p<0.05 versus venlafaxine
In this study, almost 1 in 3 (28.9%) patients were successfully treated with Brintellix® compared to 1 in 5 (19.9%) with venlafaxine.1
Patients with MDD were significantly more satisfied‡ with Brintellix® as a treatment compared to the SNRI desvenlafaxine§2
When assessing satisfaction with medication using change in quality of life enjoyment and satisfaction questionnaires (Q-LES-Q), patients with a partial response to previous selective serotonin reuptake inhibitor (SSRIs)§ were significantly more satisfied with Brintellix® as a treatment compared to the SNRI desvenlafaxine.2 All patients included in the study reported low satisfaction with their prior SSRI treatment§ (40%) at baseline.2
Change in Q-LES-Q satisfaction with medication domain score (percentage scale) at week 82
Adapted from Wang G et al. 2020
*p<0.05 versus venlafaxine
In this study, almost 1 in 3 (28.9%) patients were successfully treated with Brintellix® compared to 1 in 5 (19.9%) with venlafaxine.1
Patients with MDD were significantly more satisfied‡ with Brintellix® as a treatment compared to the SNRI desvenlafaxine§2
When assessing satisfaction with medication using change in quality of life enjoyment and satisfaction questionnaires (Q-LES-Q), patients with a partial response to previous selective serotonin reuptake inhibitor (SSRIs)§ were significantly more satisfied with Brintellix® as a treatment compared to the SNRI desvenlafaxine.2 All patients included in the study reported low satisfaction with their prior SSRI treatment§ (40%) at baseline.2
Change in Q-LES-Q satisfaction with medication domain score (percentage scale) at week 82
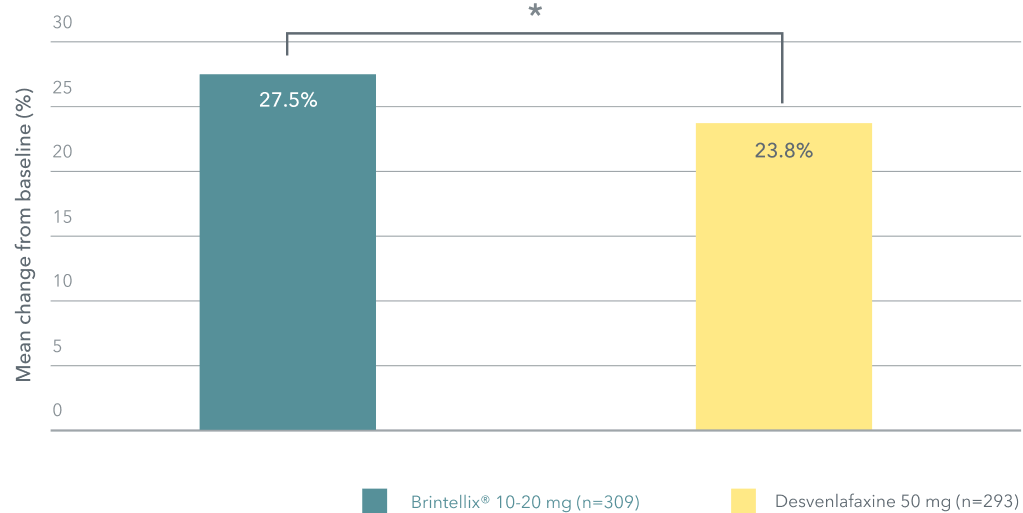
Adapted from: McIntyre RS et al. 2023.
*p=0.044.
Patients treated with Brintellix® reported significantly greater satisfaction with their medication than those who received desvenlafaxine.2 Satisfaction with antidepressant medication combines patient perceptions of treatment efficacy and tolerability and has been shown to directly correlate with treatment adherence in patients with MDD.5,6 Non-adherence to antidepressants remains a major challenge in clinical practice.7
Adapted from: McIntyre RS et al. 2023.
*p=0.044.
Patients treated with Brintellix® reported significantly greater satisfaction with their medication than those who received desvenlafaxine.2 Satisfaction with antidepressant medication combines patient perceptions of treatment efficacy and tolerability and has been shown to directly correlate with treatment adherence in patients with MDD.5,6 Non-adherence to antidepressants remains a major challenge in clinical practice.7

† As measured with successfully treated patients (STPs) at week 8. STP, defined as ≥50% reduction in MADRS total score and no TEAE. Data based on an 8-week randomised, double-blind head-to-head study
in adult Asian patients with acute MDD comparing Brintellix® (10 mg/daily) and venlafaxine XR (150 mg/daily).1
‡ As measured by the Q-LES-Q satisfaction with medication subdomain. Change from baseline to week 8 in Q-LES-Q percentage scale was 27.5% with Brintellix® and 23.8% with desvenlafaxine, p=0.044.2
§ In patients with a partial response to previous SSRI treatment: citalopram, escitalopram, paroxetine, sertraline. Data based on an open-label study (VIVRE) evaluating evaluating the efficacy of Brintellix® vs. desvenlafaxine in patients with MDD who had a partial response to SSRI treatment. The primary endpoint was a non-inferiority test in change in MADRS total score at week 8 relative to desvenlafaxine, where noninferiority was established (-0.47 advantage to Brintellix®, CI [-1.61; 0.67]).2
Abbreviations:
MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; STP, successfully treated patients; TEAE, treatment-emergent adverse event.
† As measured with successfully treated patients (STPs) at week 8. STP, defined as ≥50% reduction in MADRS total score and no TEAE. Data based on an 8-week randomised, double-blind head-to-head study
in adult Asian patients with acute MDD comparing Brintellix® (10 mg/daily) and venlafaxine XR (150 mg/daily).1
‡ As measured by the Q-LES-Q satisfaction with medication subdomain. Change from baseline to week 8 in Q-LES-Q percentage scale was 27.5% with Brintellix® and 23.8% with desvenlafaxine, p=0.044.2
§ In patients with a partial response to previous SSRI treatment: citalopram, escitalopram, paroxetine, sertraline. Data based on an open-label study (VIVRE) evaluating evaluating the efficacy of Brintellix® vs. desvenlafaxine in patients with MDD who had a partial response to SSRI treatment. The primary endpoint was a non-inferiority test in change in MADRS total score at week 8 relative to desvenlafaxine, where noninferiority was established (-0.47 advantage to Brintellix®, CI [-1.61; 0.67]).2
Abbreviations:
MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; STP, successfully treated patients; TEAE, treatment-emergent adverse event.

