As discussed in the first Part in this series, recent advances in neuroimaging and genetic testing have transformed our understanding of brain diseases. With further advances in research, tools will allow us to detect changes in the brains of patients with Alzheimer’s and Parkinson’s disease years — perhaps even a decade or more — before clinical symptoms appear. Detecting these diseases at the early stages may one day permit timely intervention and the opportunity to engage in disease-modifying interventions, thereby reducing the burden that untreated neurodegenerative disorders place on patients and their families.
How do we understand “Disease” in Neurology?
A broader understanding of disease, coupled with increasingly accessible genetic and neuroimaging assessments to identify markers of disease not visible clinically, have begun to change the way we define, approach, and understand disease in neurology. Traditionally, clinicians had no choice but to wait for patients to present with specific signs and symptoms—such as the characteristic resting tremor in Parkinson’s disease—before prescribing treatment. Neurological disorders are typically diagnosed only at stages which are already relatively advanced in terms of the underlying neurodegenerative process. For example, the classical motor features of Parkinson's disease occur when approximately 50% of dopaminergic neurons in the substantia nigra have been lost.1 Likewise, in Alzheimer’s disease, the protein plaques and tangles that are thought to result in neuronal dysfunction and cell death steadily accumulate for years before the first clinical symptoms manifest.2 Unfortunately, one doesn’t typically go to the doctor unless something is wrong!
With advances in genetic testing and neuroimaging, however, new diagnostic tools have blurred the line between disease and health, reshaped recommendations for how people in an asymptomatic or prodromal stage of disease should be managed, and even changed what it means to be a patient.3 Clinicians and researchers are increasingly able to detect risk factors for a disease that have yet to manifest in the form of symptoms (at the preclinical stage of disease) and mild markers of a disease that do not meet full diagnostic criteria (at the prodromal stage of disease). By recognizing the pre- or early-symptomatic stages of disease, we may be able to detect many neurological diseases before symptoms become evident to patients or family members. Diseases such as Alzheimer’s and Parkinson’s have an especially long lead time—a decade or more—that can be tracked using an array of quantifiable markers.1,4-6 These biological markers could allow clinicians to recognize and treat disease at earlier and earlier stages, thereby prolonging survival and improving patients’ quality of life.
Neurology and the “Pre”-Patient
The first signs of Alzheimer’s Disease
Evidence suggests that elevated amyloid-β levels in CSF, atrophy of the hippocampus, and mild cognitive impairment (MCI) can be detected up to 10 years before a formal diagnosis of Alzheimer’s disease is made.2 These discoveries are truly remarkable, and only in recent years has such information become available to researchers and clinicians. Notably, clinicians may be able to use these associative markers to identify prodromal Alzheimer’s disease and allow future patients to live longer with intact quality of life (Figure 1).7 In fact, the most recent criteria to diagnose Alzheimer's disease, developed by the National Institute on Aging and Alzheimer's Association (NIA-AA), are based on three groups of biomarkers: β amyloid deposition, accumulation of the protein tau, and neurodegeneration (the “AT(N)” classification system).8 Although the AT(N) system is designed as a research tool, it is hoped that clinical practice will continue to keep pace with scientific discovery.
Quality of life in Alzheimer's disease progression.
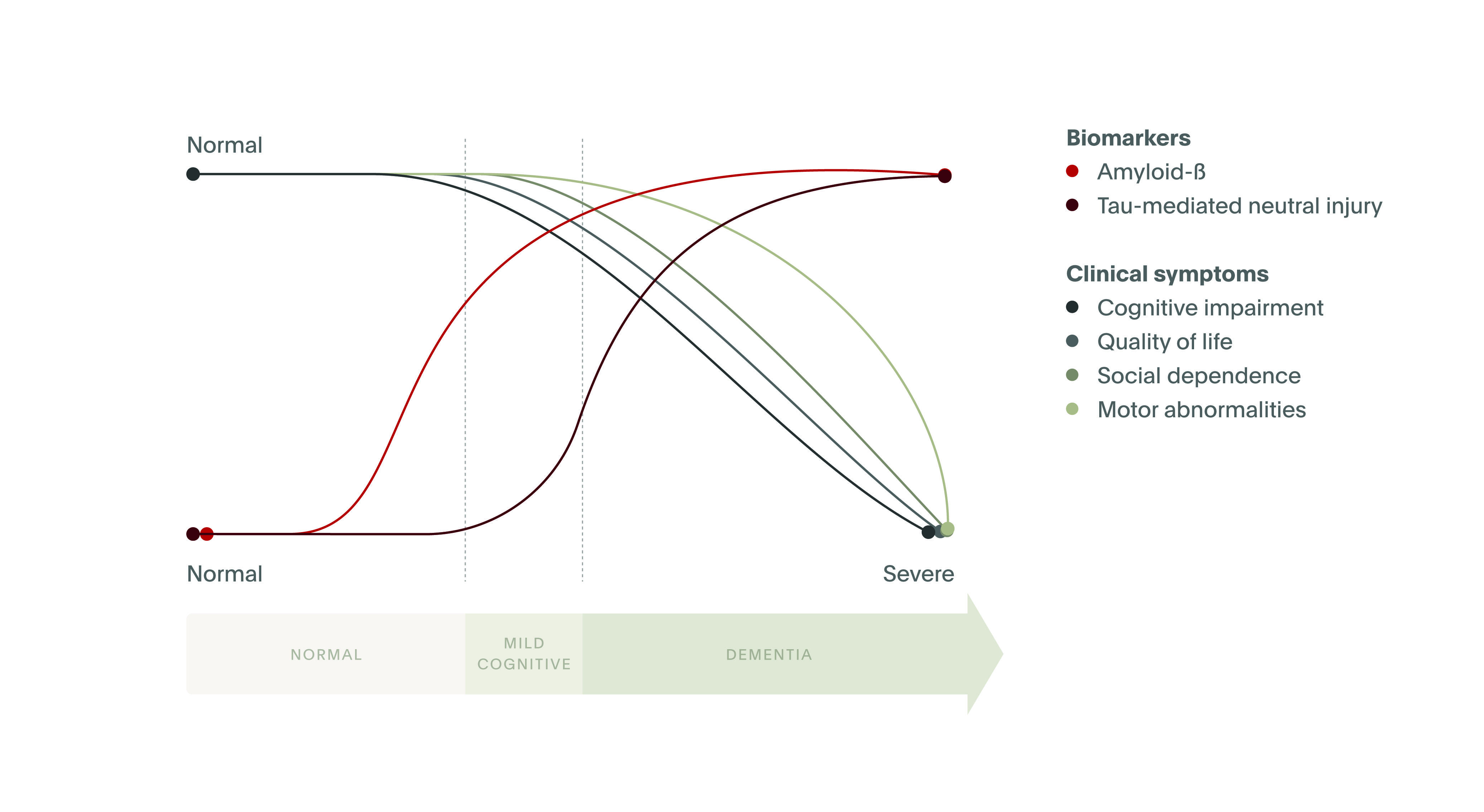
Source. Adapted from Masters et al. Nature Reviews Disease Primers, 2015.7
Source. Adapted from Masters et al. Nature Reviews Disease Primers, 2015.7
Research has found that amyloid deposition occurs alongside subtle behavioral disturbances that are often noticeable only to the patient, such as mild memory problems that occur early in Alzheimer’s disease. Specifically, patients at a preclinical or prodromal stage of Alzheimer’s disease frequently experience hypernosognosia, or an increased awareness of memory changes that precedes any clinically detectable cognitive deficits. Further, recent research has shown that for patients in the very early stages of Alzheimer’s, amyloid pathology correlates with an increased self-awareness of memory dysfunction (Vannini et al., 2017).9 As discussed below (“Screening today and tomorrow”), continued research may shine a light on additional markers that could be used to detect Alzheimer’s disease before the onset of truly debilitating symptoms.
Early signs of Parkinson’s Disease
Although motor symptoms such as bradykinesia (slowed movement) and tremor are the hallmark of Parkinson’s disease, increasing attention has been paid to a diverse array of non-motor symptoms that can be recognized early in the course of the disease - and rightly so. Many patients with early Parkinson’s report non-motor symptoms such as constipation, depression, hyposmia (loss of sense of smell), and REM sleep behavior disorder (RBD), which may be troublesome enough to impair quality of life and interfere with daily activities. Even more concerning, these non-motor symptoms are known to be markers of future disease progression.1,10 As such, even otherwise healthy patients who exhibit non-motor symptoms carry an increased risk of developing Parkinson’s later in life. Figure 2 provides a summary of these risk factors and the stage in the disease course when they appear.11
Biological markers in Parkinson’s: Preclinical, prodromal, and clinical stages
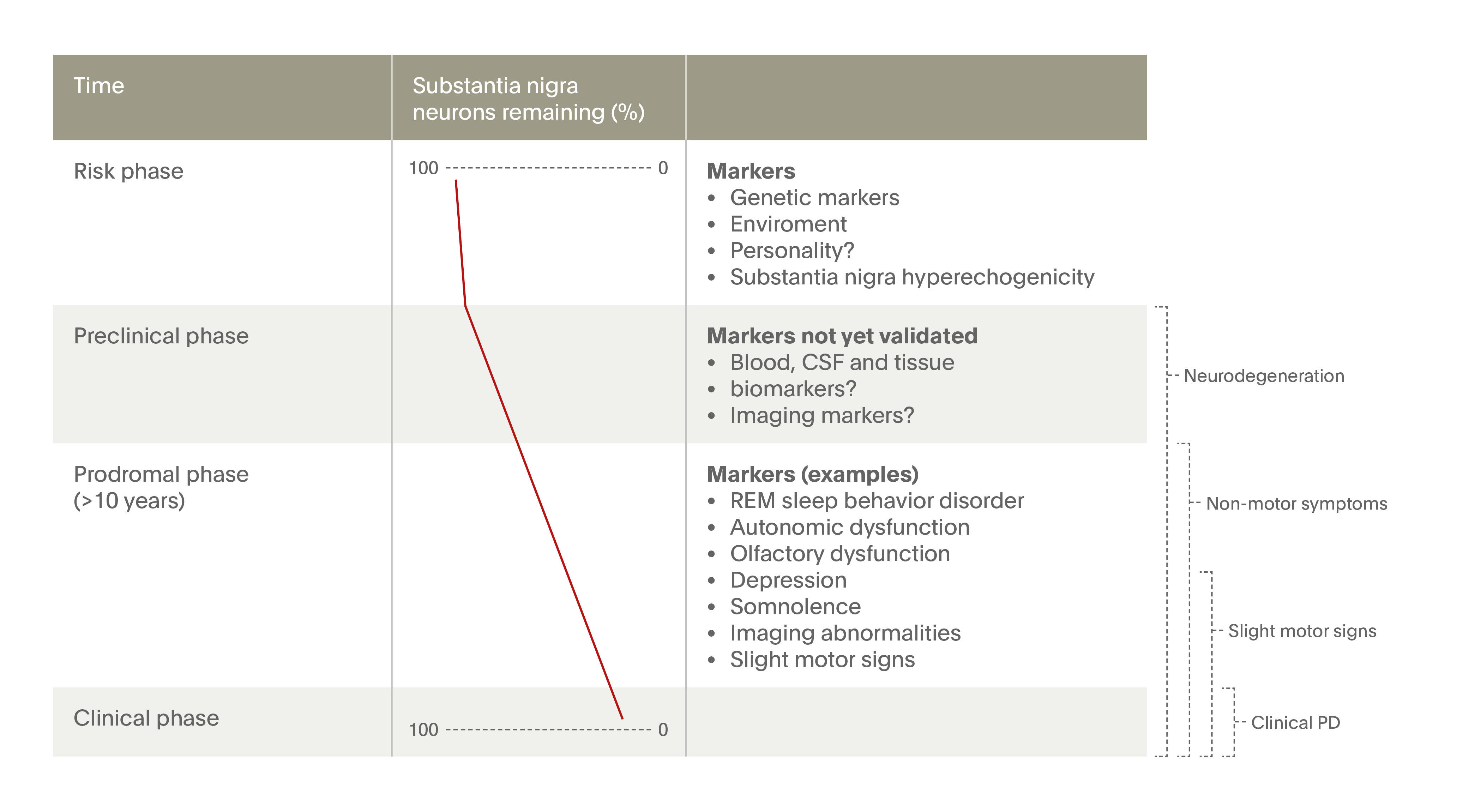
Source. Adapted from Postuma & Berg, 2016.11
Source. Adapted from Postuma & Berg, 2016.11
Given that the development of Parkinson’s disease is associated with a complex array of symptoms, screening tools with relatively high sensitivity and specificity have been used to determine which patients with prodromal symptoms are at greatest risk of disease progression.12 Because Parkinson’s is a relatively uncommon disease (lifetime risk approximately 1-2%),13 however, it is challenging for clinicians to identify whether particular symptoms are parkinsonian in origin. For example, one of the most common symptomatic changes associated with prodromal Parkinson’s is anosmia, or loss of the sense of smell. It is unclear, however, how anosmia is related to other biological changes in Parkinson’s.14 Moreover, olfactory dysfunction is common in the general population—not only in those at high risk of Parkinson’s—with up to 20% of adults experiencing an impaired sense of smell (see also “Advances in Parkinson’s disease research”). Anosmia in isolation is therefore not specific enough to make a pre-diagnosis of Parkinson’s. Other markers have been identified, including RBD, which is perhaps the strongest marker of prodromal Parkinson’s disease.15 In the next section, we discuss ongoing research that has explored the clinical utility of these and other markers of Parkinson’s disease.
Screening today and tomorrow
Neurodegenerative disorders are currently diagnosed based on the presence of specific clinical signs and symptoms that present only relatively late in the disease course. Current diagnostic criteria for Alzheimer’s disease, for example, require the presence of progressive cognitive impairment, whereas Parkinson’s disease is marked by the classic motor and non-motor manifestations described above. In the years since these criteria were established, however, our understanding of the biological basis of neurodegenerative diseases has considerably advanced. New healthcare technologies have granted researchers an unprecedented understanding of the mechanisms leading to neurodegeneration, and characterizing the earliest stages of Alzheimer’s and Parkinson’s has become the focus of intense research interest. As researchers continue to discover new predictive markers for these diseases—including characteristics detected through neuroimaging, genetic studies, and blood tests—opportunities for timely intervention are expected to improve in turn.
Genetic biomarkers
Genetic risk factors are known to increase the likelihood that one may develop Alzheimer’s disease or Parkinson’s later in life. Alzheimer’s is associated with variants in the apolipoprotein E (ApoE) E4 allele, although carrying the high-risk gene variant does not necessarily indicate that one will develop dementia.16-17 Parkinson’s disease, likewise, is linked with mutations in the LRRK2 gene, alpha-synuclein, GBA1 and several other risk loci,18 although most cases of the disease are not associated with any identifiable genetic cause. Therefore, DNA sequencing in and of itself currently has a limited ability to predict whether Alzheimer´s or Parkinson´s is in the likely medical future of individual patients.
Another disadvantage of genetic tests for Alzheimer´s and Parkinson´s is that they cannot provide any information to patients or their providers about neurodegenerative changes that may be occurring in the patient’s brain. Detecting these changes requires a different approach—one that is now being met by growing research into diagnostic imaging and laboratory tests for these disorders.
Neuroimaging biomarkers
Alzheimer’s disease is thought to be caused in part by the accumulation of two proteins, amyloid and tau, which form plaques and tangles (or bunches of twisted fibers) in the brain.19 In recent years, technologies such as MRI and positron emission tomography (PET) have allowed these neurodegenerative changes to be visualized at prodromal and even preclinical stages of disease.20-21 PET imaging, for example, has shown that cognitively normal patients with detectable levels of amyloid are more likely to develop mild cognitive impairment (MCI) than patients without this biomarker.22 Combined with other imaging biomarkers, these findings may be useful in diagnosing and staging Alzheimer’s disease.
Buildup of beta-amyloid proteins tracks the evolution of Alzheimer’s disease
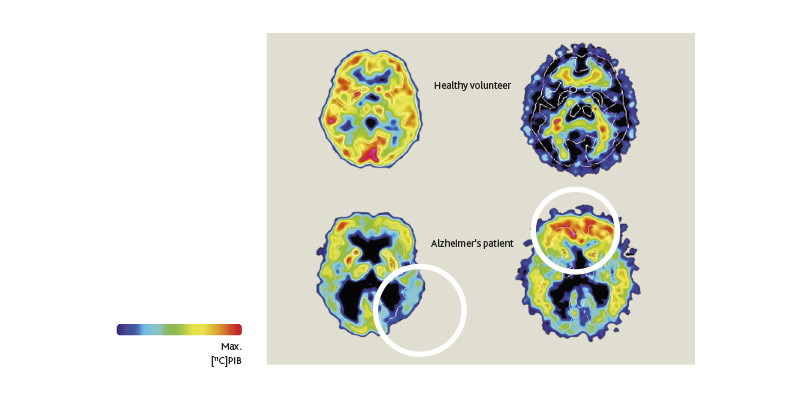
Likewise, neuroimaging has highlighted pathophysiological changes that occur in Parkinson’s disease. Parkinson’s is caused by the gradual deterioration of dopaminergic neurons in the substantia nigra, a structure responsible for the generation of movement.23 The motor symptoms of Parkinson’s, however, begin only after about 50% of these neurons have been lost.1 As in Alzheimer’s, the long prodromal phase in Parkinson’s provides opportunities for neuroimaging to facilitate early diagnosis, including at the pre-motor stage.24
One disadvantage of these neuroimaging tools is that they are expensive: a single PET scan costs up to $4000 USD and is, in many cases, not covered by health insurers. Instead, a more cost-efficient and more widely available approach may be to detect markers of disease through a simple blood test.
Peripheral biomarkers
Recent studies of Alzheimer’s disease have shown that the same amyloid proteins that accumulate in the brain can also be found in the blood of high-risk patients.25-26 These discoveries may one day make it possible to screen patients for Alzheimer’s disease in a primary care setting. In Parkinson’s disease, preliminary research suggests that blood tests could be used to distinguish the disease from other neurological problems that share some of the same clinical features.27 In recent years, peripheral tissue biopsies (e.g., of skin, salivary glands or gastrointestinal tract) to detect pathological aggregates of alpha-synuclein have also shown promise as diagnostic biomarkers.28 Although these tests are not ready for clinical use, they represent a first step toward cost-effective technologies that could be used to detect these neurodegenerative diseases prior to the onset of disabling symptoms.
Opportunities to intervene
Once detected, the early stages of Alzheimer’s and Parkinson’s disease offer a valuable opportunity for clinical intervention. Pre-patients may be able to reduce their risk of Alzheimer’s disease progression through lifestyle adjustments, such as by exercising or seeking out social or cognitive stimulation.29 Other evidence points to the importance of controlling cardiovascular risk factors in midlife—vascular problems, for example, may accelerate the buildup of amyloid plaques in the brain.30 In all, nearly one-third of Alzheimer’s cases may be preventable based on modifiable risk factors.31-32
Pharmacological treatments for Alzheimer’s disease that act early in the disease process have also become more promising. In particular, one landmark study (the “A4 Study”) is currently testing whether disease-modifying therapies can prevent cognitive decline due to Alzheimer’s in high-risk older adults who have no symptoms of disease.33 The success of these new therapeutic agents in clinical trials would make it even more important and timely to diagnose Alzheimer’s earlier and more accurately. In Parkinson's disease, evidence is sufficiently strong to recommend physical activity and, arguably, moderate doses of caffeine, for primary prevention.34 Pharmacological interventions targeting numerous pathophysiological pathways are under active investigation. Besides the role of biomarkers in identifying patients earlier in their disease course, "biomarker-driven phenotyping" in Parkinson's disease (which is heterogeneous regarding the underlying molecular pathogenesis) could be used to enrich clinical trials with biologically more homogenous subtypes which are more likely to respond optimally to therapies that affect the biological processes within each subtype (for example, where mitochondrial dysfunction or inflammation is central).35
When is Diagnosis Appropriate?
Because effective disease-modifying treatments for Alzheimer’s and Parkinson’s are not yet available, advancements in the earlier detection of disease raise a key question: how many patients would want to know whether they had a high risk of developing a neurodegenerative disorder 10, 15, or even 20 years prior to the onset of noticeable symptoms? Would you want to know, even if there was no treatment to stop the disease? The answers that most people give to these questions may surprise you. A large, global survey of more than 10,000 people conducted by a healthcare company in 2014 found that nearly 75% of people would want to know if they were prone to developing a neurological disease, even if there was no cure.36 The survey also found that an overwhelming majority of respondents (83%) thought that early detection and diagnosis was important and over half would be prepared to pay for testing themselves, even if not covered by health insurance.
Because Alzheimer’s and Parkinson’s disease are characterized by devastating neurodegenerative changes, informing someone who has yet to receive a diagnosis of either disease that he or she may experience life-changing problems in the near or distant future is a sensitive topic. Although our understanding of the risk factors linked with neurodegenerative diseases has advanced, it is important to note that not all patients experiencing these disease “markers” will in fact go on to develop disease in the future. The inherent uncertainty of preclinical diagnosis poses numerous ethical concerns, which will be discussed in Part 4 of this four-part series. Briefly, however, a few benefits and risks of early detection and management should be mentioned. On the one hand, early detection provides pre-patients with the valuable gift of time—time to make decisions about their personal and medical life while they still retain decisional capacity.37 Patients with a high likelihood of developing Alzheimer’s, for example, have time to create a living will, travel, and experience life in a manner that may not be possible after full symptom onset. On the other hand, receiving news that one is at high-risk of developing a neurodegenerative disease can cause significant psychological distress. An ongoing conversation among healthcare providers, geneticists, and patient advocacy groups is therefore needed to ensure that early diagnostic markers are used in the most appropriate and sensitive manner.
Conclusions
Earlier recognition, coupled with thoughtful and compassionate intervention, may improve the overall quality of life for patients in the preclinical or prodromal stages of disease and increase the number of years that patients live without disability. With the ability to detect markers of disease earlier and earlier prior to clinical onset, researchers have also begun examining treatments that may either stop or slow the progression or development of disease altogether. If such initiatives are successful, the world may one day see a dramatic decrease in the number of patients suffering from Alzheimer’s and Parkinson’s disease. While these advancements may still be years in the future, the ambition to prolong a healthy and fulfilling life for future patients is a worthy cause.
Acknowledgements
We would like to thank Professor John Hardy for discussing what is currently known as well as ongoing issues in preclinical and prodromal neurology which provided the foundation for this article.