Tau-PET imaging in the clinic is on the horizon and would complement amyloid-PET and structural and functional MRI in our search for tools in the differential diagnosis and staging of dementias.
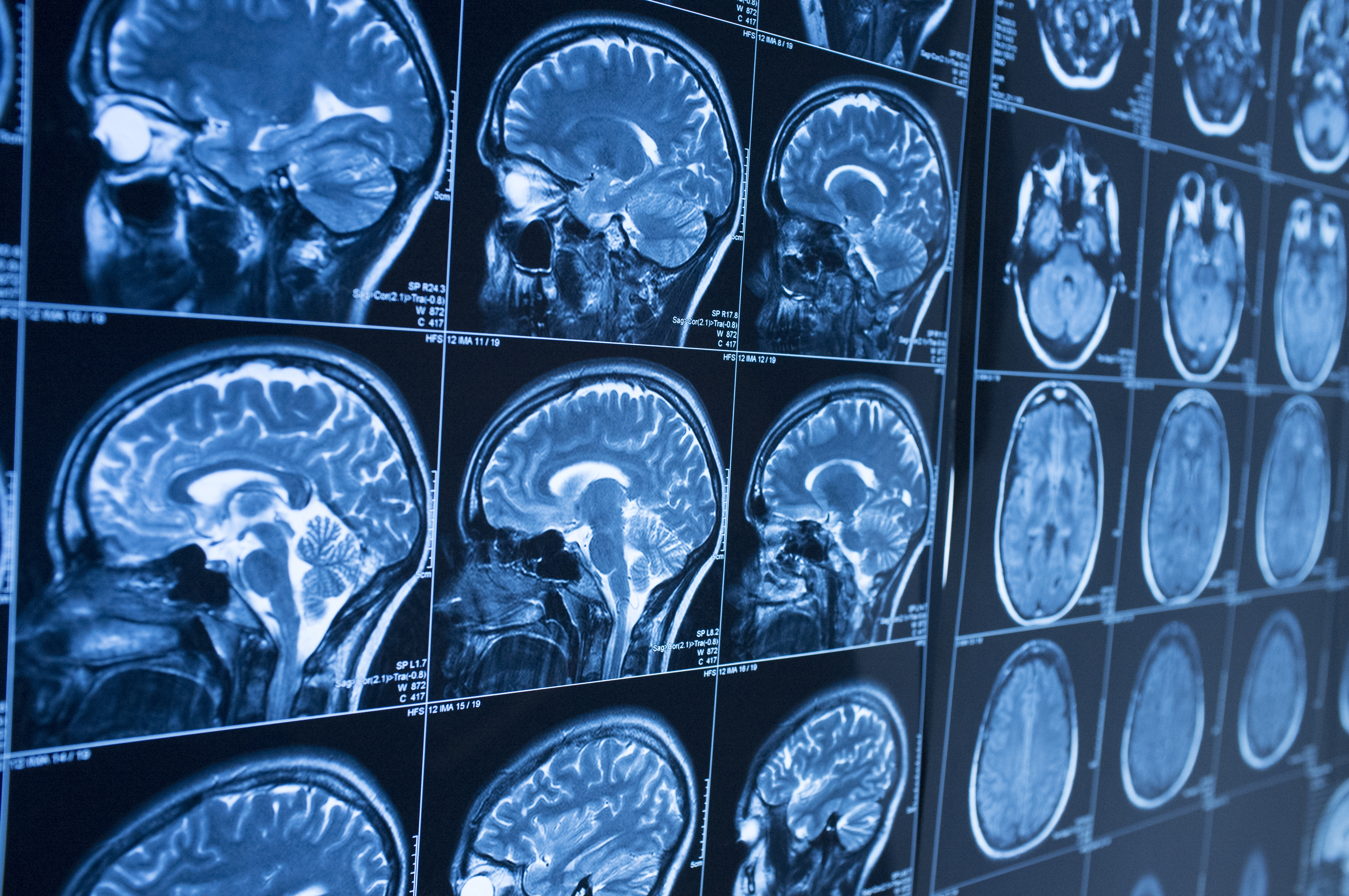
Increasing severity of Alzheimer’s disease (AD) is associated with a progressive breakdown in anatomical brain connections that can be revealed by functional MRI.1 Such use of advanced imaging to map changes in the brain connectome was among several areas of progress picked out by Massimo Filippi (San Raffaele Scientific Institute, Milan, Italy) in the EAN 2020 Virtual Congress session devoted to news in neuroimaging.
The Milan group has already shown that -- in people with a similar degree of cognitive impairment -- functional MRI allows early-onset AD to be distinguished from behavioral variant frontotemporal dementia.2
Increasing the field strength of MRI enhances detection of amyloid plaques
In other developments, use of ultra high-field MRI has now been shown to increase the detection of amyloid plaques that are evident post mortem. The ability to image pathology at high spatial resolution may offer a new means for the in vivo assessment of amyloid load in patients with AD.
Machine learning applied to MRI
Professor Filippi also drew attention to the growing role of deep learning through which artificial intelligence systems compare brain scans from normal controls and people with dementia to identify patterns that will refine diagnosis and prognosis.3
In an addition to existing knowledge about the prognostic value of structural MRI, a study from the Alzheimer's Disease Neuroimaging Initiative (ADNI) shows that annual decrease in volume predicts conversion from mild cognitive impairment to AD only when baseline volume exceeds a certain threshold. This finding has implications clinically, but also for the use of atrophy rate as an endpoint in trials.4
Tau as a driver of dementia
In the same session, Alexander Drzezga (University of Cologne, Germany) discussed advances in amyloid and tau imaging. He reviewed recent evidence that higher amyloid load in people with subjective cognitive decline is associated with faster rate of deterioration in a range of cognitive domains5 – just as it predicts rate of decline in people who already have objective cognitive impairment.
The hope is that tau-PET reveals the location of developing atrophy as well as the nature of pathology
But Professor Drzezga noted that there is a high frequency of age-related amyloid pathology irrespective of cognitive status, and that many patients in the IDEAS (Imaging Dementia—Evidence for Amyloid Scanning) study in whom AD is being considered as a diagnosis do not have amyloid on PET scanning.6
He looked forward to an era in which tau-PET, though still experimental at present, provides clinically useful information about the location of neurodegeneration and complements evidence from amyloid-PET about neuropathological processes underlying dementia.
In disease staging, tau may prove more useful than amyloid as a marker, he suggested, since amyloid accumulation -- which appears to begin far earlier than that of tau -- tends to reach a plateau before the onset of clinical progression.
For the latest updates on sea.progress.im, subscribe to our Telegram Channel https://bit.ly/telePiM
Our correspondent’s highlights from the symposium are meant as a fair representation of the scientific content presented. The views and opinions expressed on this page do not necessarily reflect those of Lundbeck.