The presence of SNP rs3892097 in the CYP2D6 gene is associated with an elevated risk of developing Alzheimer's disease (AD). Individuals with SNP rs3892097 in the CYP2D6 gene exhibit impaired drug metabolism, which can impact the selection and dosage of prescribed medications.
Cytochrome P450 2D6 (CYP2D6) is a crucial enzyme encoded by one of the twelve extensively studied functional CYP genes involved in the metabolism of a significant proportion of commonly used drugs. CYP2D6, specifically, plays a vital role in the metabolism of approximately 70-80% of drugs.1 Within the CYP2D6 gene, the presence of single nucleotide polymorphisms (SNPs), such as rs3892097, has been linked to an increased susceptibility to developing Alzheimer's disease (AD). Studies have demonstrated that individuals carrying this SNP exhibit a higher risk of AD, indicated by an odds ratio of 1.29 and a 95% confidence interval of 1.03-1.62 (p=0.026).2
CYP2D6, a drug-metabolizing enzyme expressed in the brain, plays a neuroprotective role by metabolizing endogenous neural compounds like catecholamines and deactivating neurotoxins. Studies have shown that lower levels of certain cytochrome P450 (CYP) products, including epoxide metabolites of arachidonic acid (AA) and epoxyeicosatrienoic acids (EETs), have exhibited neuroprotective effects in AD models.3 Furthermore, a clinical study observed an age-dependent increase in CYP2D6 levels, while individuals with Parkinson's disease displayed significantly lower CYP2D6 levels in specific brain regions.4 This suggests that the polymorphism of rs3892097 in the CYP2D6 gene may hinder its neuroprotective role and lead to AD.
The association between AD and the variant CYP2D6 has been a subject of interest in scientific research. CYP2D6 is a gene responsible for encoding an enzyme that plays a crucial role in drug metabolism, including medications commonly used to treat AD, such as donepezil.5 Studies have suggested that variations in the CYP2D6 gene may contribute to differences in the metabolism and response to certain medications used in AD treatment. potentially influencing drug efficacy and adverse effects.6 Furthermore, clinical studies have demonstrated that individuals carrying the CYP2D6 rs3892097 variant exhibit poor metabolization7, indicating potential implications for drug metabolism and response.
In a prospective study, it was observed that individuals with the rs3892097 variant exhibited increased plasma levels of risperidone and its metabolite.8 This finding was further supported by another study involving amlodipine medication, which showed similar results in individuals with this variant.9 The elevated plasma levels can be attributed to the impaired metabolism of drugs in poor metabolizers. As a result, the drugs are not efficiently broken down and eliminated from their system, leading to their accumulation in the bloodstream. On the one hand, it may enhance the drug's efficacy, but on the other hand, it raises the risk of adverse effects and potential toxicity. Therefore, it is essential to closely monitor drug plasma concentrations in poor metabolizers to adjust dosage regimens appropriately and ensure the safety and effectiveness of drug therapy.
The CYP2D6 gene plays a significant role in AD. Variations in this gene are associated with an increased risk of developing AD and impaired drug metabolism, which can impact the effectiveness of medications and lead to potential side effects (Figure 1). Understanding CYP2D6 variants can enhance personalized approaches to AD treatment. Further research is necessary to uncover the underlying mechanisms and explore potential therapeutic strategies.
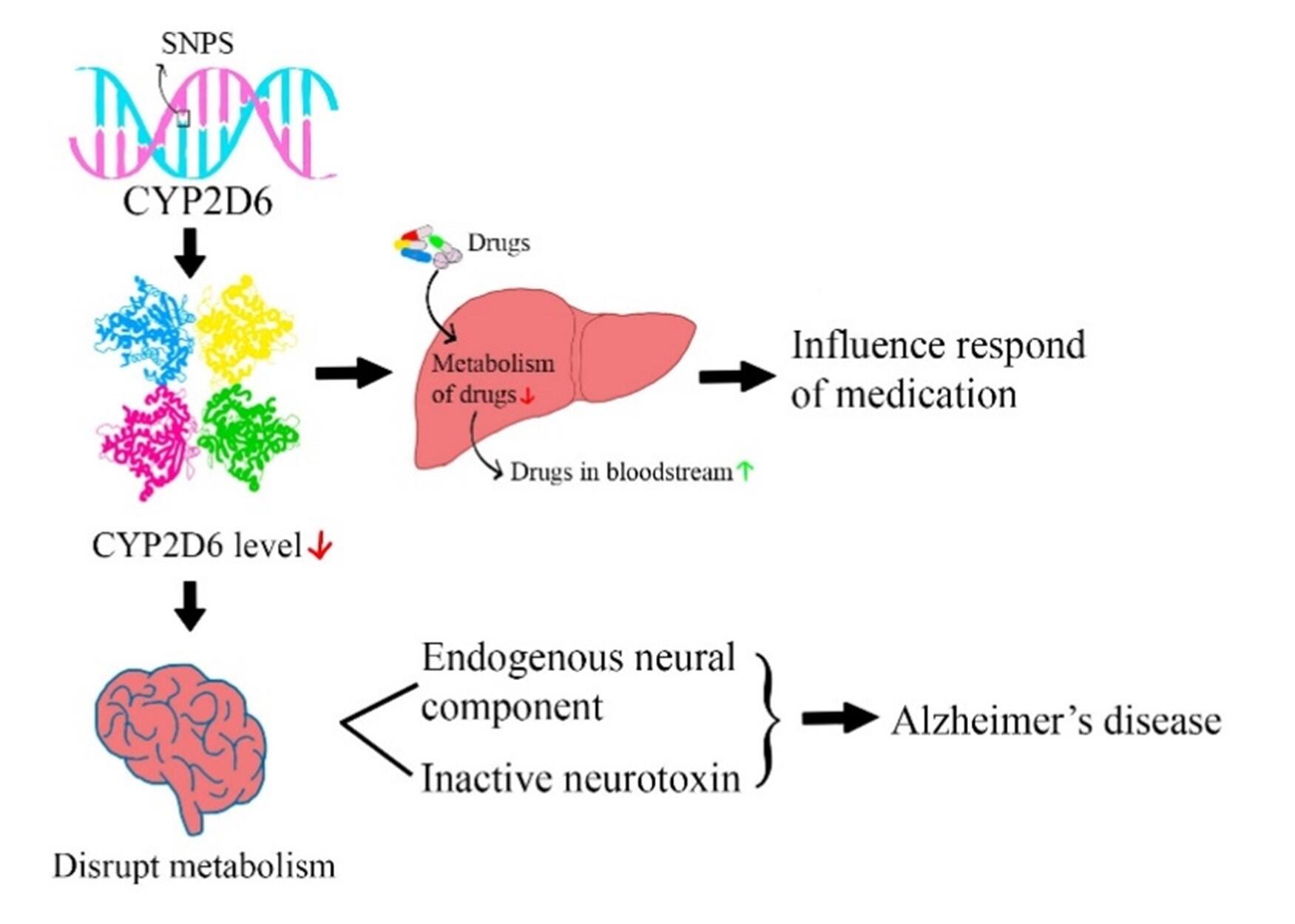
Figure 1. Effect of SNPs in risk of developing AD and impaired drug metabolism. The presence of SNPs in the CYP2D6 gene, specifically rs3892097, has been associated with an increased risk of developing Alzheimer's disease (AD). This particular SNP is known to cause a reduction in the levels of CYP2D6, both in the brain and the liver. The presence of rs3892097 is associated with poor metabolism, leading to decreased drug activation. This can have implications for the response to medications such as donepezil, where the concentration of the drug may increase due to the impaired metabolism. In the brain, lower levels of CYP2D6 result in a decreased breakdown of endogenous neural components and reduced inactivation of neurotoxins. These factors ultimately contribute to the development of Alzheimer's disease. The presence of the SNP rs3892097 highlights the role of impaired drug metabolism and its association with the risk of developing AD (summarized and adapted from 1-9).
This article was authored by Valentinus Besin, MD, PhD and Farizky Martriano Humardani, B.Med
Figure 1. Effect of SNPs in risk of developing AD and impaired drug metabolism. The presence of SNPs in the CYP2D6 gene, specifically rs3892097, has been associated with an increased risk of developing Alzheimer's disease (AD). This particular SNP is known to cause a reduction in the levels of CYP2D6, both in the brain and the liver. The presence of rs3892097 is associated with poor metabolism, leading to decreased drug activation. This can have implications for the response to medications such as donepezil, where the concentration of the drug may increase due to the impaired metabolism. In the brain, lower levels of CYP2D6 result in a decreased breakdown of endogenous neural components and reduced inactivation of neurotoxins. These factors ultimately contribute to the development of Alzheimer's disease. The presence of the SNP rs3892097 highlights the role of impaired drug metabolism and its association with the risk of developing AD (summarized and adapted from 1-9).
This article was authored by Valentinus Besin, MD, PhD and Farizky Martriano Humardani, B.Med